Temperature control for spindle assembly studies in fission yeast
S. pombe is a very powerful organism to study microtubules assembly. The easy manipulation of fission yeast, the short dividing time and the existence of temperature-sensitive mutant strains allows to dissect the role of microtubules in various aspects of S. pombe biology. Temperature is a controlling factor of microtubule assembly.
CherryTemp meets cell biology studies requirements. It reaches below ambient temperatures for efficient microtubules depolymerization or endocytosis arrest, it shifts from 5 to 45C in less than 10 seconds and thus prevents gradient formation inside the sample, it uses electronic control for steady temperature control and microfluidic technology to prevent fluid-flow shear stress on biological samples, it fits on any microscope setting. Data presented here have been published by Velve-Casquillas et al., 2010 in Lab on a Chip.
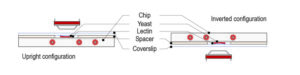
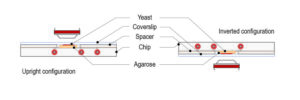
Temperature control of spindle assembly experimental set-up
CherryTemp temperature controller device: Our PDMS chip is composed of microchannels allowing the circulation of thermalized fluid. The chip is connected to Peltier devices set at two different hot/cold temperatures making possible to quickly change from low to high temperature. Our chip is mounted on a thin glass coverslip,that comes on top of the biological sample, spacers can be used depending on sample thickness. In our system, the fluid is never in contact with the sample thus preventing any shear stress
Cell imaging and microscopy Microtubule depolymerization experiments were performed on cdc25-22 cells expressing GFP-Atb2 tubulin. Mitotic spindle experiments were done using temperature sensitive cut7-24ts expressing GFP-Atb2. Images were acquired using a Yokogawa CSU-10 spinning disc scan head on a Nikon TE2000e inverted microscope. 3D imaging was done using a 100/1.45 NA objective, Metamorph 7.5 software for microscope and Hamamatsu ORCA-AG-CCD camera control.
Ultra fast temperature shift device for in vitro experiments under microscopy
Temperature control for spindle assembly studies in fission yeast
Reversible ultra-fast heat-induced microtubules depolymerization
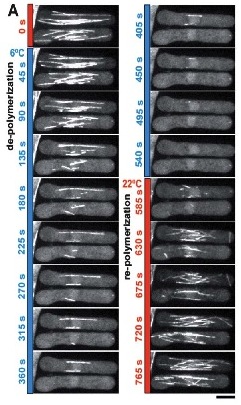
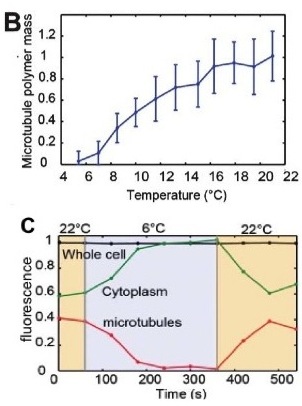
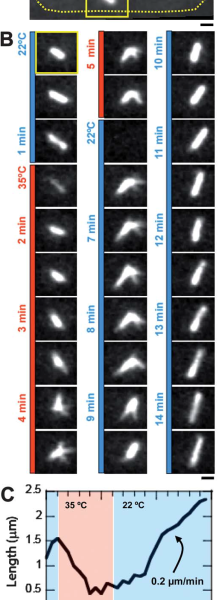
To validate the efficacy of CherryTemp, we used GFP-Atb2 tubulin expressing yeast and follow fluorescence in vivo. At 22C, microtubules exhibit a typical elongated shape (Fig1A). At 16C, we could already quantify a decrease in microtubule polymer mass (Fig1B), and we observed a complete depolymerization when cells were at 6C (Fig1A and 1B) with a loss of microtubule-associated fluorescence signal (Fig1A) and an increase in cytoplasmic-associated fluorescence (Fig1C). This suggests that tubulin dimers were released from the microtubules and move to the pool of free-tubulin dimers. We found that the time to reach full depolymerization was cell-size dependent. This process was reversible, and microtubules repolymerize when we shifted back the temperature from 6C to 22C. Using our temperature controller, we could efficiently monitor cold-induced microtubule depolymerization and re-polymerization while at the microscope stage.
Reversible ultra-fast heat-induced spindle assembly phenotype
We next wanted to validate the induction of temperature-sensitive mutant phenotype using our device. To this aim, we used Cut7-25ts expressing GFP-Atb2 yeast. Cut7 is a kinesin protein, involved in mitotic spindle formation. At restrictive temperature Cut7-25ts mutant do not form bipolar elongated spindle. At permissive temperature (22C) bipolar spindle is correctly formed and visible with GFP-Atb2 protein (Fig 2A and B). Few minutes after we shifted to 35C, the restrictive temperature, the bipolar spindle retracted and adopted a monopolar shape (Fig2B), this was characterized by a shortening in spindle length (Fig2C). When we shifted the temperature back to permissive level, we could revert the spindle phenotype (Fig2B) and retrieve original spindle length after a few minutes (Fig2C).
Microtubules and cellular processes
Microtubules are polymeric and very dynamic structures, which are part of the cytoskeleton of every cells. They play a crucial role in various cellular processes. During cell division they are important for chromosomes segregation in mitosis. They also ensure intra-cellular transport, nucleus and organelles movement. They participate in cell polarity and cellular migration. Microtubules, along with intermediate filament and actin filament can be seen as the bone and muscle of a cell, they really define cell architecture and shape. The role of microtubules in S.pombe cell division, growth and polarity has been very well documented (S.Martin, 2009; Piel and Tran, 2009).
Microtubules structure
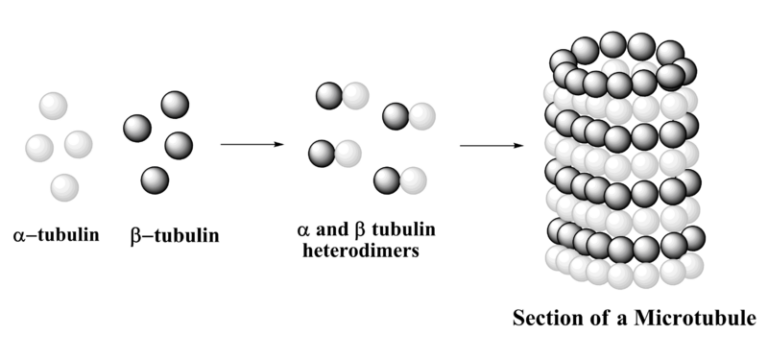
A microtubule is a hollow cylinder and a polarized non-covalent polymer, with a plus and a minus end. A microtubule is composed of 13 protofilaments, each of them composed of alfa and beta-tubulin heterodimers. There are three phases in microtubule assembly: a nucleation, an elongation and an equilibration phases. The nucleation phase consists in the formation of the alfa and beta-tubulin dimers. This phase is called nucleation as alfa and beta dimers are the “nucleus” chore components of microtubules. In S.pombe, microtubule nucleation occurs in perinuclear microtubules organizing center called spindle pole body. The elongation phase is a growing phase, during which tubulin dimers are added to the microtubule plus end allowing microtubules to elongate away from the MTOC. Finally when the concentration of polymerized actin is equivalent to that of cytoplasmic free actin, the microtubule has reached an equilibration phase.
Microtubules depolymerization is controlled by temperature
Microtubules are very dynamic structures, with a constant addition and removal of tubulin dimers at the microtubule plus end tip. When tubulin dimers depolymerize and microtubule start to shrink this is refer to as “catastrophe”. The switch from a catastrophe phase to a growing phase is called “rescue”phase.
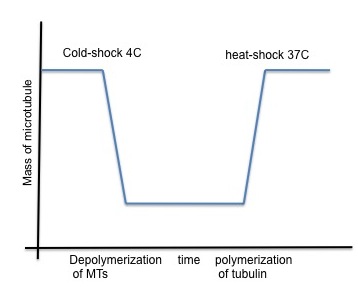
A number of drugs can interfere with microtubule polymerization, among them colchicine has been widely used in laboratory. Interestingly, once microtubules are formed, their stability becomes temperature dependent (Lodish, 2000). Subjecting the cells to low temperature (6C) is enough to obtain microtubule depolymerization, along with re localization of microtubule-associated protein to the perinuclear domain. This cold-shock is reversible and with heat microtubules can polymerize again.
Interestingly, fishes swimming in cold water, posess tubulin which is cold resistant and can polymerize at very low temperature (-1.8C).
References
C.Fu, J J. Ward, I.Loiodice, G.Velve-Casquillas, FJ. Nedelec, and PT. Tran, Phospho-Regulated Interaction between Kinesin-6 Klp9p and Microtubule Bundler Ase1p Promotes Spindle Elongation, Developmental cell, 2009
S. Castagnetti, S. Oliferenko, P. Nurse Fission Yeast Cells Undergo Nuclear Division in the Absence of Spindle Microtubules, PLoS Biology, 2010
S. Martin Microtubule-dependent cell morphogenesis in the fission yeast, trends in Cell Biology, 2009
H.Lodish, Molecular Cell biology, WH Freeman edition.
M.Piel and PT. Tran Cell Shape and Cell Division in Fission Yeast , Current Biology 2009
S. Ben-Aroya, X. Pan, JD. Boeke, and P. Hieter, Making temperature-sensitive mutants, Methods Enzymol. 2010
G. Velve Casquillas, C. Fu, M Le Berre, J. Cramer, S. Meance, A. Plecis, D. Baigl, JJ. Greffet, Y. Chen, M. Piel and PT. Tran Fast microfluidic temperature control for high resolution live cell imaging, Lab on a chip 2010