Immobilization of C. elegans during live cell imaging: rational
Although they have proved valuables, all of these procedures show a number of limitations. The use of chemical compounds, induces physiological modifications, which may interfere with the biological question studied. Also, with these techniques, C. elegans worms do not have identical orientation, they may not be easily accessible for further manipulations. While the use of polysterene nanoparticles is easy to set-up, one caveat is that immobilization is dependent on worm growth stages (Kim et al., 2013).

Ultra fast temperature shift device for in vitro experiments under microscopy
Microfluidic device active methods
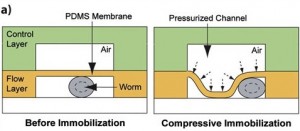
1. The Compressive method. This technique takes advantage of the physical properties of PDMS membrane. In this setting, the microfluidic device contains integrated valves. The C. elegans worm is placed in the bottom flow layer channel, with applied-pressure the membrane is deflected and as a result the worm is physically immobilized. A pressure adjustment allows the use of different worm developmental stages. Pressure applied immobilization may not be compatible with a long immobilization period. Furthermore, the caveats with this technique is that it is not user friendly, it is tricky to generate and the set-up unless one establishes a collaboration with a well trained microfluidic research group. For biologists users, we recommend the use of the passive method, which is more easy to handle.

2. Microfluidic temperature induced immobilization. This method described in Chung et al., 2008 allows a strict immobilization of C. elegans, stopping all internal movement and thus allowing sub-cellular structures imaging. In this setting (see figure) the worm is placed in the bottom flow channel (red), two micro valves control the flow and the worm placement (green), and a temperature-controlled liquid flow channel is placed on top of the worm channel allowing a rapid cool-down of C.elegans temperature. While this method is very useful, it implies a total immobilization of the worm and hence it is not adequate for live-animal video imaging. However, with appropriate devices temperature cool down can be reverse. Also, the use of a worm sorter may prove necessary to get the worms in the same orientation.
C. elegans Immobilization using microfluidic devices: a time-saving strategy!
Here, we present the two main microfluidics strategies to immobilize worms for microscopy imaging : a passive and easy to handle method, in which the worm is trapped in a worm clamp, and an active method in which the worm is immobilized using valves-applied pressure or cold.
The main reason why experimentator will want to use microfluidics is for time-saving considerations and reproducible immobilization conditions. Microfluidics allows a fast handling and consistent immobilization of C. elegans. (San-Miguel A and Lu H, 2013). Moreover, microfluidic immobilization presents several advantages among which unidirectional orientation, no superposition of worms, further manipulation can be performed: such as drug injection or ultra fast temperature control of C. elegans worm environment.
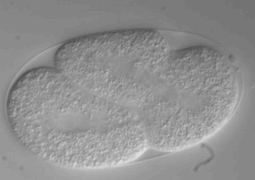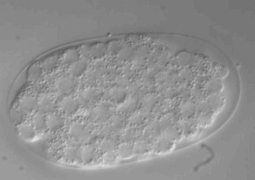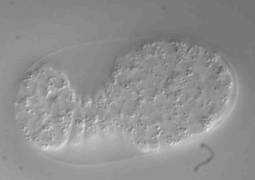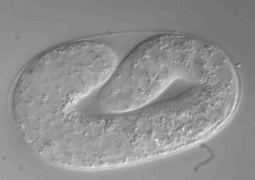
Microfluidic passive method : the worm trap
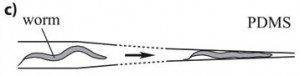
This method is based on a mechanical trapping of C. elegans. The microfluidics chip is composed of parallel microchannels, this is very convenient since it allows high-throughput C. elegans processing. Through action of liquid flow, the worm enters a microchannel, whose width is reduced to a point to that the worm is immobilized (see figure). One advantage of this method is that upon flow reversion, the worm can be retrieved, and drugs can be delivered through the liquid flow and worm can be oriented in the same direction. However, one has to notice that residual movement may occur with this method but it does not appear to be limiting since it can be compensated with appropriate Imaging software (San Miguel and Lu, 2013, wormbook). This method is of particular interest since it can be combined with worm environment manipulation: drugs injection or ultra fast temperature shift.
Conclusion : C.elegans immobilization
Using microfluidic devices for C. elegans immobilization is time saving: with rapid handling, immobilization and imaging time spent per worm experimentation. It allows you to eliminate the use of anesthetics, which perturbed animal physiology. Depending on your needs, you may want to choose an easy to set up method like microfluidic worm traps, or if you need to have a complete block of both external and internal worm movement you’ll find that cooling device may be more suitable.
References
Aufderheide, KJ. (2008). An overview of techniques for immobilizing and viewing living cells. Micron 39, 71–76
Kim E, Sun L, Gabel CV, Fang-Yeng C. (2013). Long-term imaging of Caenorhabditis elegans using nanoparticle-mediated immobilization. PLoS ONE 8(1): e53419
San-Miguel A and Lu H , Microfluidics as a tool for C. elegans research*(September 24, 2013), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.7.1.
Hulme, S.E., Shevkoplyas, S.S., Apfeld, J., Fontana, W., and Whitesides, G.M. (2007). A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab Chip7, 1515-1523.
Chokshi, T. V., Ben-Yakar, A., and Chronis, N. (2009). CO2 and compressive immobilization of C. elegans on-chip. Lab Chip 9, 151-157.
Chung, K., Crane, M.M., and Lu, H. (2008). Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637-643.
S. Ben-Aroya, X. Pan, JD. Boeke, and P. Hieter, Making temperature-sensitive mutants, Methods Enzymol. 2010