Abstract
Introduction
Cytokinesis is a cellular process in which a cytoplasm and chromosomes of a single cell are divided to generate two daughter cells. This mechanism is well conserved through evolution and requires the interaction between myosin motor II proteins with actin filaments. This contractile ring will form at the site of cell division and will constrict the cell as to form two daughter cells. The formation and position of this ring, is ensured by a set of specialized proteins, more than 130 genes are involved in the ring complex, and has been extensively studied, notably in S. pombe.
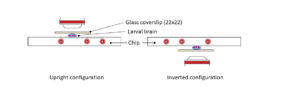
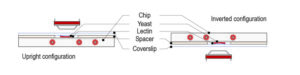
Ultra fast temperature shift device for in vitro experiments under microscopy
Cytokinesis in S. pombe
Scientists have been focusing on deciphering the positioning, assembling, maintenance and constriction of the ring, leading to the actual separation of the two daughter cells.
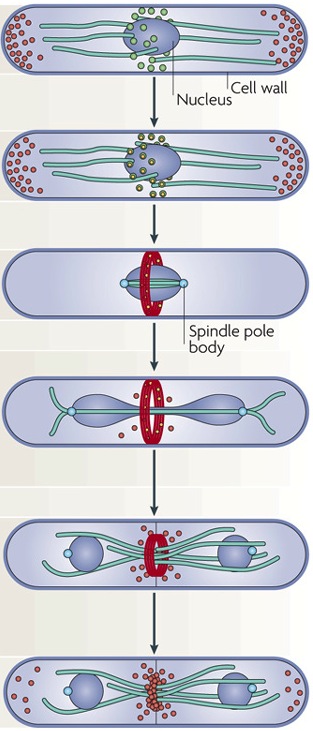
The mid1p protein is a key actor in the positioning of the ring. During interphase, mid1p is located in structures called nodes. These nodes exchange positional cues with the cell nucleus and are located at the cortico-medial area of the cells. Positioning of the ring is governed by dual permissive and restrictive positional information. There are inhibiting mechanisms which prevent the formation of the ring at the cell tip. Notably, the pomp1p kinase acts to exclude mid1p from localizing at the cell end tip (Bathe and Chang, 2010).
During the early phases of mitosis, more proteins are recruited to the nodes, namely: cdr1, cdr2, wee1. Cdr1 and cdr2 kinases make a link between ring formation and progression of the cell through the cell cycle. Indeed, they inhibit wee1, a kinase responsible for keeping the cell in G2 phase, hence releasing the cell for entry into mitosis.
Progressing through cell cycle, the nodes maturate with the addition of nuclear and cytoplasmic Mid1p, myosinII, Rng2, F-BAR, cdc12 proteins (Pollard and Wu, 2010). At maturation, Cdc12, a formin protein, interacts with profilin, to polymerize actin filaments. These actin filaments elongate along the plasma membrane and make contact with other nodes. Following actin filament formation, nodes start to move, condense and form a continuous ring. This node movement is ensured by the interaction between node myosin II protein and actin filament. At this point, the ring actin filaments become contractile and start to constrict. The mechanism leading to the disassembly of the ring is not known. However after total ring constriction and disassembly, a septum starts to form leading to the plasma membrane fission.
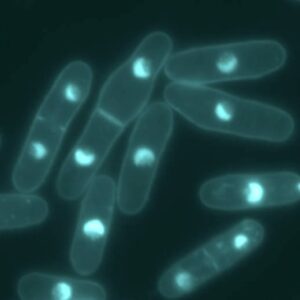
Using live-cell imaging and fluorescent-tagged protein to study fission yeast cytokinesis
The combination of fluorescent-tagged proteins with high-resolution imaging allows very precise anlayses of cytokinesis events. With such techniques, Pollard’s group has produced a series of very elegant articles. Notably, they decipher the identity and origin of interphase nodes (Akamatsu et al., 2014). They found that , during interphase,there are two types of nodes, namely type1 and 2. During early mitosis the two types superpose and segregate at the end of mitosis. Using a series of fluorescent-tag cdr1p, cdr2p, mid1p to mark type 1 nodes and blt1p, gef2p, klp8p for type2, they could follow the assembly and disassembly of these proteins during the cell cycle. Moreover, they use temperature-sensitive mutant yeast to investigate the role of protein regulators, such as sid2 kinase, on node formation and dispersal (Akamatsu et al., 2014). Thus, they very precisely and in a time-fashion manner describe the series of cellular events leading to, and regulating cytokinesis (Wu et al., 2003; Akamatsu et al., 2014; Stachowiak et al., 2014)
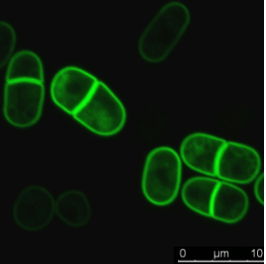
Combine fast temperature shift and cytokinesis live imaging observation
Researchers have been able to describe cytokinesis proteins assembly and acto-myosin movement minute per minute. In their review, Pollard and Wu, 2010 explain that at the time of ring maturation, nodes condense by moving at around 30 nanometers per second over a 10 min period. What if you could use temperature-sensitive mutant to activate and inactivate node protein during this time frame? CherryTemp is a temperature controller that allows you to shift yeast temperature (5 to 45 C) in seconds and combine it with live-cell imaging. Now, you can track your fluorescent protein and subject them to permissive or restrictive temperature in seconds, or do cycle of temperature-shift experiments. CherryTemp allows you to access a new level of control on your proteins and go further in cytokinesis understanding.
References
- M.Bathe and F.Chang Cytokinesis and the contractile ring in fission yeast: towards a systems-level understanding, Trends Microbiol, 2010
- TD. Pollard and JQ. Wu Understanding cytokinesis: lessons from fission yeast Nat Rev Mol Cell Biol, 2010
- M. Akamatsu, J.Berro, KM Pu, IR. Tebbs,and TD. Pollard Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase , JCB, 2014
- MR. Stachowiak, C. Laplante, HF. Chin, B. Guirao, E. Karatekin,TD. Pollard, and B. O’Shaughnessy ,Mechanism of Cytokinetic Contractile Ring Constriction in Fission Yeast, Dev cell, 2014
- JQ Wu, JR. Kuhn,DR. Kovar, and Thomas D. Pollard Spatial and Temporal Pathway for Assembly and Constriction of the Contractile Ring in Fission Yeast Cytokinesis , Dev Cell, 2003