Engineered C. elegans as a model organism for a better understanding of type 2 diabetes in humans
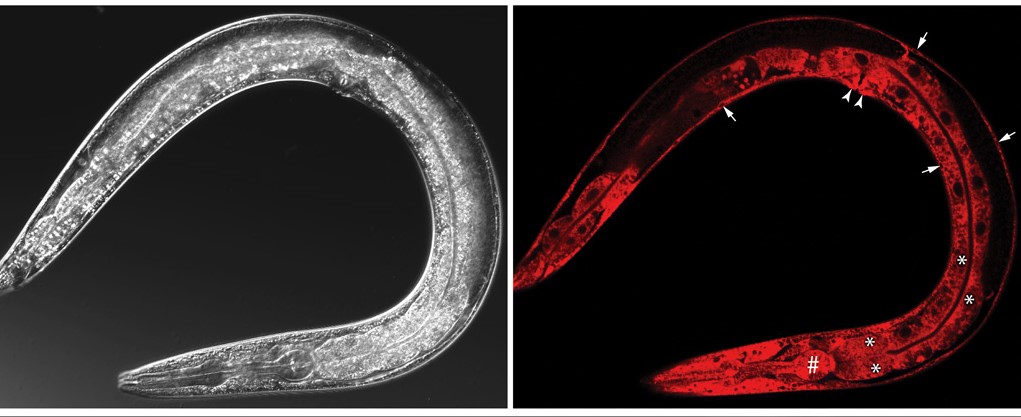
To better understand the mechanisms of peptide hormones, Xin Li et al. performed a research on translocon associated protein α (TRAPα). They reveal a primordial role of TRAPα in the biosynthesis of insulin. They have used C. elegans as a model organism for mammals to better understand the pathogenesis of type 2 diabetes. The insulin secretion pathway analysis demonstrates the importance of the endoplasmic reticulum translocation machinery in insulin biogenesis. To further extend these findings in rats and humans, they experimented in pancreatic β cells of rats where TRAPα would directly promote preproinsulin translocation, influences proinsulin maturation & insulin secretion. All these advances conduct to suggestions that preproinsulin translocation and proinsulin trafficking could lead to the development of type 2 diabetes.
Ultra fast temperature shift device for in vitro experiments under microscopy
Abstract
“The mechanistic basis for the biogenesis of peptide hormones and growth factors is poorly understood. Here, we show that the conserved endoplasmic reticulum membrane translocon-associated protein α (TRAPα), also known as signal sequence receptor 1, plays a critical role in the biosynthesis of insulin. Genetic analysis in the nematode Caenorhabditis elegans and biochemical studies in pancreatic β cells reveal that TRAPα deletion impairs preproinsulin translocation while unexpectedly disrupting distal steps in insulin biogenesis including proinsulin processing and secretion. The association of common intronic single-nucleotide variants in the human TRAPα gene with susceptibility to type 2 diabetes and pancreatic β cell dysfunction suggests that impairment of preproinsulin translocation and proinsulin trafficking may contribute to the pathogenesis of type 2 diabetes.”