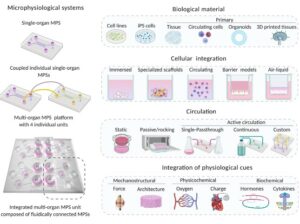
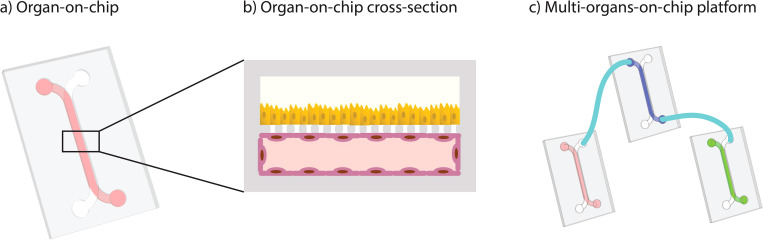
Introduction
Many Organ-on-Chip platforms are still not robust for all cell types, are not reproducible from experiment to experiment or user to user, and should be independently qualified as fit for purpose. Furthermore, they are not always compatible with end-user lab workflows. These and other barriers must be overcome before OoC models can be adopted by the industry and accepted as animal alternatives by regulators.
The government regulations regarding Microphysiological system platforms should take into consideration the complexity of models in addition to promoting compatibility to make them more user-friendly. Regulators being involved in the process of development of Microphysiological systems is key as the end goal of the development of these models is to replace animal models and regulators have direct overview of it.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The authors state that “Organ-on-chip (OoC) and multi-organs-on-chip (MOoC) systems have the potential to play an important role in drug discovery, disease modeling, and personalized medicine. However, most devices developed in academic labs remain at a proof-of-concept level and do not yet offer the ease of use, manufacturability, and throughput that are needed for widespread application.
Commercially available OoC is easier to use but often lacks the level of complexity of the latest devices in academia. Furthermore, researchers who want to combine different chips into MOoC systems are limited to one supplier, since commercial systems are not compatible with each other.
Given these limitations, the implementation of standards in the design and operation of OoCs would strongly facilitate their acceptance by users. Importantly, the implementation of such standards must be carried out by many participants from both industry and academia to ensure widespread acceptance and adoption.
This means that standards must also leave room for proprietary technology development next to promoting interchangeability. An open platform with standardized interfacing and user-friendly operation can fulfill these requirements. In this Perspective article, the concept of an open platform for OoCs is defined from a technical perspective. Moreover, we discuss the importance of involving different stakeholders in the development, manufacturing, and application of such an open platform.”
References
Vollertsen AR, Vivas A, van Meer B, van den Berg A, Odijk M, van der Meer AD. Facilitating implementation of organs-on-chips by open platform technology. Biomicrofluidics. 2021 Oct 12;15(5):051301. DOI: 10.1063/5.0063428. PMID: 34659603; PMCID: PMC8514251.