Why switching from conventional models to Organs on Chips?
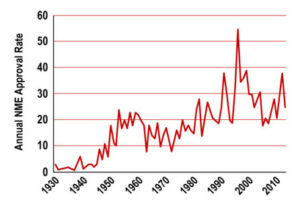
Whereas 2D culture models are able to provide large amounts of relatively inexpensive data, this type of model weakly represent the complex pathophysiology in patients. In addition, they often require computational modeling and systems biology approaches to predict in vivo drug responses ; thus the need for organs on chips.
Biomimetic 3D tissue models recapitulate the complex interactions between different types of cells in vivo that are mediated by various soluble and insoluble factors such as cytokines, nutrients, growth factors, hormones, the extracellular matrix and intercellular junctions, all of which can be controlled within the organ-on-a-chip which is clinically more relevant than in 2D culture models.Organs on chips and drug development
Organs on chips also have advantages over animal models. Although animal surrogates to human diseases can emulate the real physiological complexity of a whole organism, they are nowadays facing the skepticism of scientists and reglementary agencies regarding their translatability to humans.
Some of the microdevices features for organ-on-chips represent key advantages over both cellular and animal models. Indeed, the microdevice is optically transparent allowing high-resolution analysis, compartmentalized channel designs for the multi-organs chip, the capability to control microenvironment factors and to sample the media for the identification of novel biomarkers.Organs on chips and drug development
For all these reasons, and for the evaluation of drug efficacy and safety, organs-on-chips provide compelling advantages over other in vitro and in vivo experimental models.
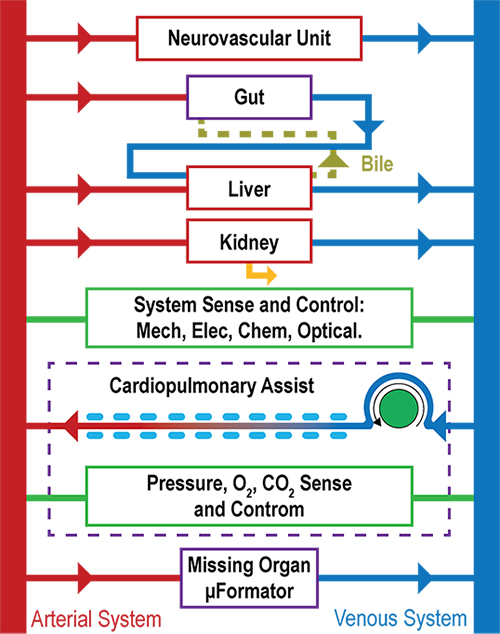
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Potential uses in drug screening
The Organ-on-a-chip technology is being used to develop cost-effective in vitro models for hit-to-lead and lead optimization that can more reliably predict the efficacy, toxicity, and pharmacokinetics of drug compounds in humans.
-Efficacy: It is a key factor during clinical trials. A poor efficacy is often caused by an absence of suitable prediction for the therapeutic effect of the drug. It has been shown that a spheroid (3D organoids) culture of lung cancer is far more efficient in the prediction of the effective drug concentration in humans than a classical 2D culture. This survey included screening experimentation of 12 drugs designed to inhibit the epithelial-mesenchymal transition (EMT) (1). Organs on chips and drug development
-Toxicity screening: Unexpected adverse drug effects are the second most common cause of clinical trial failures and are also responsible for the costly withdrawals of marketed drugs. For example, organ-on-chips have already proved their ability to significantly reduced the biological noise inherent to in vivo metabolomic models (2) or their ability to emulate the contractile function of the heart muscle and thus prevent drug-induced cardiotoxicity. Organ-on-a-chip models can, therefore, be used for accurate prediction and mechanistic investigation of dose-limiting human toxicities of prospective drugs, as well as for the exploration of new therapeutic approaches to mitigate the observed toxic effects.
-Pharmacokinetics of drug: Several studies have demonstrated the potential of organ-on-chip microdevices to model key pharmacokinetic processes that govern drug bioavailability, with a strong emphasis on drug metabolism (4-6). These studies suggest that organ-on-chip technology could be used to develop novel assays to simulate and predict critical physiological responses involved in drug bioavailability, efficacy, and toxicity. The trend to move towards the body-on-a-chip model will further increase the relevance of organ-on-chip approaches.
Further applications in drug development
In addition to improving research and development efficiency in general, organ-on-a-chip platforms could be useful to support and accelerate efforts in rare diseases, stratified medicine, and nanomedicine, fields that are attracting industrial and public interest.
-Rare diseases: The development of new drugs for rare diseases is greatly hampered by the lack of appropriate preclinical models and the scarcity of patient populations available for clinical trials. Therefore, organ-on-chips might be a good solution. Some examples: heart-on-a-chip models (based on iPS cells) to study weakness of cardiomyocytes in Barth syndrome (7) or model of hereditary hemorrhagic telangiectasia, using a microfluidic device to engineer 3D vascular tissue (blood vessel-like tubular structures).
-Stratified medicine: The practice of developing drugs for particular patient populations — for example, stratified on the basis of the presence of a particular biomarker linked to disease or drug response — has become increasingly prevalent in recent years. To this end, the organ-on-a-chip technology has been applied to develop in vitro models that reflect genetic underpinnings of variability in human drug responses. The combination of organs-on-chips and iPS cells may further provide a relatively inexpensive method to generate patient- and population-specific model platforms that could be used to test drugs targeting specific mutations or polymorphisms.
-Nanomedicine: The novelty of nanotechnology approaches raises concerns regarding the potential adverse effects of the nanomaterials involved. So, the development, optimization, and clinical translation of nanomedicines require a rigorous preclinical assessment of drug-induced toxicity as well as efficacy, but these studies are challenging because of the lack of test platforms that incorporate sufficient human-relevant physiological complexity for reliable prediction of drug effects. To provide proof of principle for using organ-on-a-chip approaches to meet the needs for inexpensive, predictive models in nanomedicine, Kim et al. used a blood vessel-on-a-chip to study the translocation of lipid–polymer hybrid nanoparticles across a microfluidic endothelium mimicking the dysfunctional endothelial barrier of atherosclerosis.
References
1) Aref AR, et al. Screening therapeutic EMT blocking agents in a three-dimensional microenvironment. Integr Biol (Camb). 2013; 5:381–389. [PubMed: 23172153]
2) Snouber LC, et al. Metabolomics-on-a-chip of hepatotoxicity induced by anticancer drug flutamide and its active metabolite hydroxyflutamide using HepG2/C3a microfluidic biochips. Toxicol Sci. 2013; 132:8–20
3) Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013; 13:3599–3608.
4) LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012; 42:501–548.
5) Chao P, Maguire T, Novik E, Cheng K-C, Yarmush M. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol. 2009; 78:625–632.
6) Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012; 12:2165–2174.
(7)Wang G, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature Med. 2014; 20:616–623.