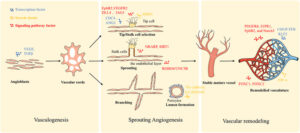
Brain: No-human in-vivo model
The human brain is one of the most convoluted organs. Contemporaneously, in-vivo and in-vitro (organotypic brain cultures for example) non-human models are unraveling the mechanism of the complex brain signaling and additionally are being used to identify the therapeutic agents.
Several vertebrates including zebrafish, mice, and rat are applied to prototype human neurodegeneration. Neuro-disorders are either replicated chemically or genetically imitated through mutation in selected no-human models. However, many drug contenders failed to meet the translation from no-human to human model after the effective screening in the animal. Often, no-human models partially exhibit the etiology and symptomatic traits of stimulated or mutated diseases. As the anatomy, physiology, and biochemistry of the human brain are substantially different from the modeled vertebrates, henceforth the comparison concerning no-human and human model is impractical.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
In-vitro analysis of human brain cells: clinical applications
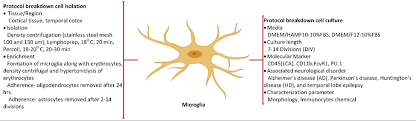
Generally, in-vitro humanmodels encompass the individual or mixed culture of primary brain cells separated from different age human or preserved cell lines. Human brain cell culturing involves rigorous and laborious biological protocols. The protocols have been devised in two phases: 1) protocol cell isolation includes the cell dissociation measures from sliced tissue, 2) protocol cell culture relates to the developing etiquettes for the considered neurological disorder. Referenced studies correlated with brain cell culture are being utilized to unveil the processes including inflammation, neurotoxicity, and neuroprotection, additionally, to screen for pre-clinical drugs to treat neurodegenerative disorders.
Limitations of dissociate human culture compensated with slice culture
The chief disadvantage of the dissociate culture is associated with brain anatomical and cellular interactions, which cannot be replicated easily. Moreover, the number of autopsies is declined day by day; hence, the availability of the brain tissue is limited. In this scenario, more often, researchers find that the yield of brain cells is relatively low after tissue dissociation.
In contrast, the advancement in slice culture method enables limited anatomical and cellular interactions and offers a model nearer to the in vivo situation. Additionally, Verwer 2002 claims that brain tissue obtained from an autopsy can be retained for a more extended period (up to 78 days), and further can be manipulated.
Organotypic brain cultures: Apprehension from upcoming technologies
Further modification in slice culture method will deliver a robust system for analyzing the fundamental biology of human brain cells and their wirings. The system will enable the understanding of basic procedures of brain cell death, cell repairing, and testing novel protocols for brain disorders. Slice culture can be utilized to explore electrical properties and synaptic functioning of the human brain via applying electrophysiological approaches. These cultures could be established as standard studies to unfold mechanisms of neuroinflammation, the death of human neurons, and glial scar formation.
Discover our brain project !
References
- Aderem A, and Underhill DM. Mechanisms of phagocytosis in macrophages. Annual Review of Immunology1999;17:593–623.
- Brewer GJ, et al. Culture and regeneration of human neurons after brain surgery. Journal of Neuroscience Methods 2001;107:15–23.
- Gonzalez-Martinez JA, et al. Neurogenesis in the postnatal human epileptic brain. Journal of Neurosurgery2007;107:628–35.
- Konishi Y, et al . Isolation of living neurons from human elderly brains using the immunomagnetic sorting DNA-linker system. American Journal of Pathology2002;161:1567–76.
- McKhann GM,et al. The isolation of neurons from normal and abnormal human cerebral cortex. Archives of Neurology1969;20:542–7.
- Ridet JL, et al. Transplantation of human adult astrocytes: efficiency and safety requirements for an autologous gene therapy. Journal of Neuroscience Research2003;72:704–8.
- Verwer RW, et al. Cells in human postmortem brain tissue slices remain alive for several weeks in culture. FASEB Journal2002;16:54–60.
- Walsh K, et al. Human central nervous system tissue culture: a historical review and examination of recent advances. Neurobiology of Disease2005;18:2–18.
- Wroblewska Z, et al. Human brain in tissue culture. II. Studies of long-term cultures. Journal of Comparative Neurology 1975;161:307–16