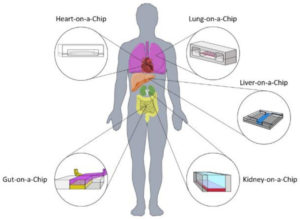
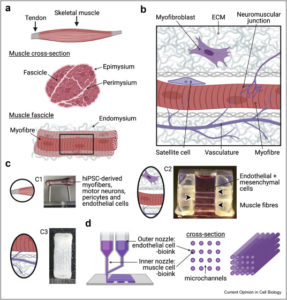
Why a muscle on a chip?
FUN FACT: Skeletal muscles account for 40% of the total body weight in humans and consume about 30% of energy intake.
Skeletal muscles are also called striated muscle. Excessive loss of muscle mass often comes with chronic pathologies, aggravating the general condition of the patient, which can result in cachexia. Most musculoskeletal disorders do not have curative treatment yet. Some diseases such as amyotrophic lateral sclerosis, Duchenne’s disease, or Lou Gehrig’s disease still do not have an effective treatment.
In parallel, some treatments of chronic diseases (diabetes, cholesterol) have undesirable effects on the striated muscles. Having access to a muscle on a chip would investigate the occurrence of such adverse effects and a better prediction of the effects of a drug candidate during the clinical phase.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
What has already been achieved with muscle-on-a-chip?
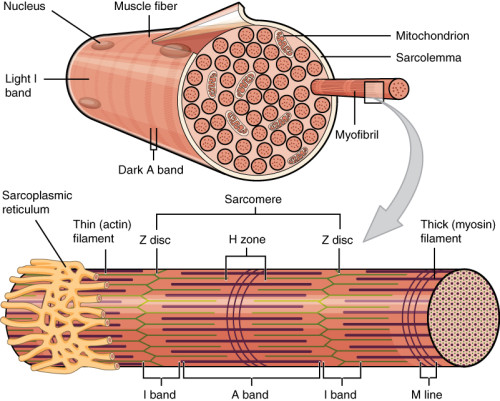
Preamble: The striated muscle cell (rhabdomyocytes) is multinucleated and rich in mitochondria. The myosin and actin proteins are responsible for contraction, under the dependence of an inflow of intracellular calcium.
A team from the University of Duke (Truskey George A) recreates a muscle unit by culturing human muscle cells taken from volunteers (1). By application of an electrical stimulus, they showed that this muscle on a chip contracts in proportion to the intensity of the current.
Moreover, they showed that the contraction passes through the release of calcium, exactly as in physiological conditions.
This short video about organs on a chip shows the spontaneous and induced rhythmic contractions of two muscles on a chip models.
A team from MIT created a neuro-muscular unit-on-a-chip (2). The neurons have been genetically engineered to respond to luminous stimuli, which once stimulated will excite the muscle cells that will contract. The rhabdomyocytes and neurons originate from mouse embryonic cells, which have been differentiated in a 3D hydrogel.
A group from the Brown Medical School Hospital (Vandenburgh H) studied Duchenne disease by reconstructing a muscle-on-a-chip from isolated myoblasts of MDX mice (genetically modified to have Duchenne myodystrophy) (3). The bio-artificial muscles are grown in 3D in a gel reproducing the extracellular matrix in a PDMS chip. This system is semi-automated, and therefore potentially compatible with high-throughput screening techniques.
Researchers from the Wyss Institute (Parker KK) developed muscle-on-a-chip applied to the tongue (4). This original approach makes possible the investigation of the muscular mechanisms involved in Duchesne’s disease. The authors showed that the muscle fibers of patients with Duchenne disease were smaller, and had a different polarization than healthy rhabdomyocytes. This results in fewer contraction capabilities of the muscles.
What's next in the muscle on a chip field?
The future in organs on a chip dedicated to muscle mainly concerns connection to other organs via the development of vessels with a contraction capacity to have the most complete vision possible of the distribution of a molecule in the human body.
References
1: Elife. 2015 Jan 9;4:e04885. doi: 10.7554/eLife.04885. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N.
2: Sci Adv. 2016 Aug 3;2(8):e1501429. doi: 10.1126/sciadv.1501429. eCollection 2016 Aug. Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Uzel SG, Platt RJ, Subramanian V, Pearl TM, Rowlands CJ, Chan V, Boyer LA, So PT, Kamm RD
3: FASEB J. 2009 Oct; 23(10): 3325–3334. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. Herman Vandenburgh, Janet Shansky, Frank Benesch-Lee, Kirsten Skelly, Janelle M. Spinazzola, Yero Saponjian, and Brian S. Tseng
4: J Cell Biol. 2016 Oct 10;215(1):47-56. Epub 2016 Oct 3. A human in vitro model of Duchenne muscular dystrophy muscle formation and contractility. Nesmith AP, Wagner MA, Pasqualini FS, O’Connor BB, Pincus MJ, August PR, Parker KK.