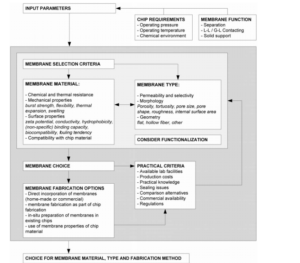
Introduction
The researchers created a microfluidic organ-on-chip solution with integrated electrochemical microsensor arrays enabling compartmentalized matrix-based organoid culture from single stem cells and real-time recording of metabolic data.
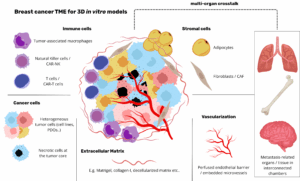
These patient-derived stem cells are an attractive model for testing drug efficacy and tolerance to personalized chemotherapy because they mimic the individual patient’s tumor in vitro. Culture conditions and cell metabolism are not accessible on a pericellular level in many organ-on-chip systems, and therefore cannot be manipulated or confirmed in situ.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The author states that “Three-dimensional cell cultures using patient-derived stem cells are essential in vitro models for more efficient and individualized cancer therapy. Currently, culture conditions and metabolite concentrations, especially hypoxia, are often not accessible continuously and in situ within microphysiological systems.
However, understanding and standardizing the cellular microenvironment are the key to successful in vitro models. We developed a microfluidic organ-on-chip platform for matrix-based, heterogeneous 3D cultures with fully integrated electrochemical chemo- and biosensor arrays for the energy metabolites oxygen, lactate, and glucose. Advanced microstructures allow straightforward cell-matrix integration with standard laboratory equipment, compartmentalization, and microfluidic access.
Single, patient-derived, triple-negative breast cancer stem cells develop into tumor organoids in a heterogeneous spheroid culture on-chip. Our system allows unprecedented control of culture conditions, including hypoxia, and simultaneous verification by integrated sensors.
Beyond previous works, our results demonstrate precise and reproducible on-chip multi-analyte metabolite monitoring under dynamic conditions from a matrix-based culture over more than one week. Responses to alterations in culture conditions and cancer drug exposure, such as metabolite consumption and production rates, could be accessed quantitatively and in real-time, in contrast to endpoint analyses.
Our approach highlights the importance of continuous, in situ metabolite monitoring in 3D cell cultures regarding the standardization and control of culture conditions, and drug screening in cancer research. Overall, the results underline the potential of microsensors in organ-on-chip systems for a successful application, e.g. in personalized medicine.”
References
Dornhof J, Kieninger J, Muralidharan H, Maurer J, Urban GA, Weltin A. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip. 2021 Dec 1. doi: 10.1039/d1lc00689d. Epub ahead of print. PMID: 34851349.
FAQ
A microfluidic organ-on-chip solution was created. It has integrated electrochemical microsensor arrays. This setup allows for compartmentalized, matrix-based organoid culture to be grown from single stem cells. It also permits the real-time recording of metabolic data. Three-dimensional cell cultures using patient-derived stem cells are considered very important in vitro models. They are used for developing more efficient and individualized cancer therapy. The new system was developed to monitor these cultures. It provides a high degree of control over culture conditions, including hypoxia. These conditions can be checked at the same time by the integrated sensors. This continuous monitoring is valuable for standardizing 3D cell cultures and for drug screening in cancer research.
Patient-derived stem cells are seen as an attractive model for specific tests. They are used for testing drug efficacy. Tolerance to personalized chemotherapy is also tested with these cells. This is because the models replicate the individual patient’s tumor in vitro. Such 3D cultures are considered necessary in vitro models for more effective cancer therapy. In one application of the new system, single, patient-derived, triple-negative breast cancer stem cells were used. These cells developed into tumor organoids on the chip. This shows the system’s ability to support complex, heterogeneous 3D cultures. Using patient-specific cells in a controlled system is believed to be beneficial for applications in personalized medicine.
In many current organ-on-chip systems, culture conditions and cell metabolism are not available for measurement at the pericellular level. Therefore, these factors cannot be manipulated or confirmed in situ. It is common for metabolite concentrations, especially hypoxia, to be inaccessible for continuous, in situ measurement within microphysiological systems. This is a problem because understanding and standardizing the cellular microenvironment is a central part of successful in vitro models. The new platform was developed to solve this. It provides continuous, in situ metabolite monitoring. This is different from traditional endpoint analyses, where measurements are only taken at the conclusion of the experiment.
The microfluidic platform has fully integrated electrochemical chemo- and biosensor arrays. These sensors are designed to measure specific energy metabolites. The metabolites monitored are oxygen, lactate, and glucose. The system allows for precise and reproducible monitoring of these multiple analytes on the chip. This monitoring can be done for over one week, even under dynamic conditions, from a matrix-based culture. By measuring these factors, responses to alterations in culture conditions can be accessed. Exposure to cancer drugs can also be studied. For example, metabolite consumption and production rates can be measured in real-time. This capability shows the promise of microsensors in organ-on-chip systems.