Introduction
In vitro generation of perfusable 3D microvessels is an important goal for tissue engineering and reliable modeling of blood vessel function. In vitro blood vessel models have yet to accurately reproduce the dynamics and responses of endothelial cells in order to grow perfusable and functional 3D vascular networks.
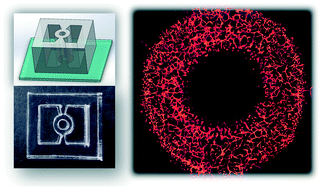
In this paper, the author talks about different vascularization mechanisms including vasculogenesis and angiogenesis to create a microfluidic device for a 3D vascularized organ-on-a-chip, 3D perfusable microvasculature inside a tissue chamber with various shapes under different microenvironment factors. Read the paper below to learn more.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The author states that “Reconstruction of 3D vascularized microtissues within microfabricated devices has rapidly developed in biomedical engineering, which can better mimic the tissue microphysiological function and accurately model human diseases in vitro.
However, the traditional PDMS-based microfluidic devices suffer from the microfabrication with complex processes and usage limitations of either material properties or microstructure design, which drive the demand for easy processing and more accessible devices with a user-friendly interface. Here, we present an open microfluidic device through a rapid prototyping method by laser cutting in a cost-effective manner with high flexibility and compatibility.
This device allows highly efficient and robust hydrogel patterning under a liquid guiding rail by spontaneous capillary action without the need for surface treatment. Different vascularization mechanisms including vasculogenesis and angiogenesis were performed to construct a 3D perfusable microvasculature inside a tissue chamber with various shapes under different microenvironment factors.
Furthermore, as a proof-of-concept, we have created a vascularized spheroid by placing a monoculture spheroid into the central through-hole of this device, which formed angiogenesis between the spheroid and microvascular network.
This open microfluidic device has great potential for mass customization without the need for complex microfabrication equipment in the cleanroom, which can facilitate studies requiring high-throughput and high-content screening.”
References
Li Q, Niu K, Wang D, Xuan L, Wang X. Low-cost rapid prototyping and assembly of an open microfluidic device for a 3D vascularized organ-on-a-chip. Lab Chip. 2021 Sep 28. doi: 10.1039/d1lc00767j. Epub ahead of print. PMID: 34581377.