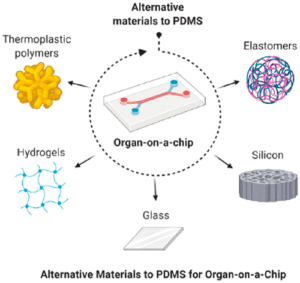
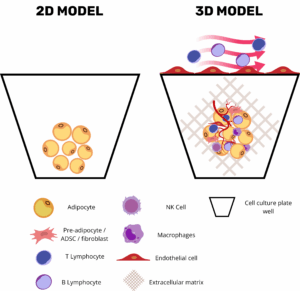
Introduction
Microfluidic 3D Endothelium-on-a-Chip – The endothelium is a thin membrane that lines the heart and blood vessels. Endothelial cells secrete chemicals that regulate vascular relaxation and contraction, as well as enzymes that regulate blood clotting, immunological function, and platelet adhesion (a whitish material found in the blood).
Endothelial dysfunction has been found to be important in predicting stroke and heart attacks due to the arteries’ failure to expand completely. Excessive blood pressure, diabetes, high cholesterol, and smoking may all contribute to the malfunction.
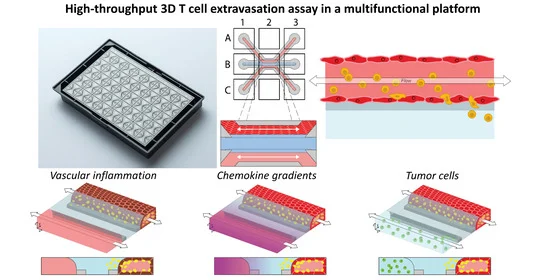
Find out in this article below how the authors use a Microfluidic 3D Endothelium-on-a-Chip to Study Transendothelial Migration and what future it holds for Organ-on-a-chip.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The author states that “The recruitment of T cells is a crucial component in the inflammatory cascade of the body. The process involves the transport of T cells through the vascular system and their stable arrest to vessel walls at the site of inflammation, followed by extravasation and subsequent infiltration into tissue.
Here, we describe an assay to study 3D T cell dynamics underflow in real-time using a high-throughput, artificial membrane-free microfluidic platform that allows unimpeded extravasation of T cells. We show that primary human T cells adhere to endothelial vessel walls upon perfusion of microvessels and can be stimulated to undergo transendothelial migration (TEM) by TNFα-mediated vascular inflammation and the presence of CXCL12 gradients or ECM-embedded melanoma cells.
Notably, migratory behavior was found to differ depending on T cell activation states. The assay is unique in its comprehensiveness for modeling T cell trafficking, arrest, extravasation, and migration, all in one system, combined with its throughput, quality of imaging, and ease of use. We envision routine use of this assay to study immunological processes and expect it to spur research in the fields of immunological disorders, immuno-oncology, and the development of novel immunotherapeutics.
References
de Haan L, Suijker J, van Roey R, Berges N, Petrova E, Queiroz K, Strijker W, Olivier T, Poeschke O, Garg S, van den Broek LJ. A Microfluidic 3D Endothelium-on-a-Chip Model to Study Transendothelial Migration of T Cells in Health and Disease. Int J Mol Sci. 2021 Jul 30;22(15):8234. doi: 10.3390/ijms22158234. PMID: 34361000; PMCID: PMC8347346.
FAQ
The endothelium is described as a thin membrane. It lines the heart and blood vessels. Chemicals are secreted by endothelial cells to regulate vascular relaxation and contraction. Enzymes are also produced that regulate several functions, including blood clotting, immunological response, and the adhesion of platelets. Endothelial dysfunction has been noted for its role in predicting heart attacks and strokes. This dysfunction is defined as the failure of arteries to expand completely. It may be contributed to by several factors, such as excessive blood pressure, diabetes, smoking, or high cholesterol.
The recruitment of T cells is a key component of the body’s inflammatory cascade. This process is what the assay is designed to study. It involves the transport of T cells through the vascular system. At a site of inflammation, a stable arrest to the vessel walls occurs. This is followed by extravasation, which is the crossing of the vessel wall. Subsequent infiltration into the tissue then happens. The described assay is used to observe these T cell dynamics in three dimensions. This observation can be done in real-time and under flow conditions. The system is a microfluidic device that is free of artificial membranes. Unimpeded extravasation of T cells is permitted by this design.
It was shown that primary human T cells adhere to endothelial vessel walls. This occurs upon the perfusion of the microvessels. The T cells can then be stimulated. Transendothelial migration, or TEM, is induced. This stimulation is achieved through vascular inflammation mediated by TNF$\\alpha$. The presence of one of two other factors is also required. These factors are either CXCL12 gradients or melanoma cells embedded in the extracellular matrix. A further observation was made. Migratory behaviour was found to be different. These differences depended on the activation states of the T cells.
The assay is noted for its ability to model several processes in one system. T cell trafficking, arrest, extravasation, and migration are all included. The system is described as having good imaging quality and being straightforward to operate. It is intended for regular use in the study of immunological processes. Research is expected to be furthered in several areas. These areas include immunological disorders and immuno-oncology. The creation of new immunotherapeutics is also an expected area of research that will be aided by this assay.