Introduction
Sexual reproduction relies on meiosis, a specialized type of cell division, which generates haploid germ cells or gametes. The genome size reduction that occurs during gametogenesis involves two successive cell divisions, termed meiosis I and II, preceded by a single round of genome replication (Dumont and Brunet, 2010). Chromosome gain or loss during meiosis generates aneuploid embryos after fertilization. Aneuploidy is hence a major obstacle in achieving reproductive success, as the vast majority of embryos formed from aneuploid oocytes are non-viable, leading to miscarriage (Nagaoka et al., 2012).
Abstract
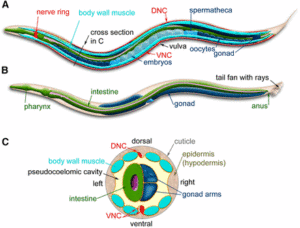
In most species, oocytes lack centrosomes. Accurate meiotic spindle assembly and chromosome segregation – essential to prevent miscarriage or developmental defects – thus occur through atypical mechanisms that are not well characterized. Using quantitative in vitro and in vivo functional assays in the C. elegans oocyte, we provide novel evidence that the kinesin-13 KLP-7 promotes destabilization of the whole cellular microtubule network. By counteracting ectopic microtubule assembly and disorganization of the microtubule network, this function is strictly required for spindle organization, chromosome segregation and cytokinesis in meiotic cells. Strikingly, when centrosome activity was experimentally reduced, the absence of KLP-7 or the mammalian kinesin-13 protein MCAK (KIF2C) also resulted in ectopic microtubule asters during mitosis in C. elegans zygotes or HeLa cells, respectively. Our results highlight the general function of kinesin-13 microtubule depolymerases in preventing ectopic, spontaneous microtubule assembly when centrosome activity is defective or absent, which would otherwise lead to spindle microtubule disorganization and aneuploidy.