Organs-on-Chip for Drug Development
A drug should pass the preclinical evaluation phase to be FDA-approved. This relies (i) on in vitro cell culture platforms and (ii) on in vivo animal models and (iii) on the clinical evaluation phase (human subjects).
Many drugs fail in clinical trials, leading to an incredibly low 1:10,000 ratio for a drug to be FDA approved and go on the market. As a consequence, each single drug development costs up to 12 years with an average of 1.7 billion USD per clinical applicable drug.
The impact of drug candidates on the human immune system could be responsible for this huge failure gap between preclinical and clinical studies. On the first hand, none of the existing in vitro platforms can emulate the cellular microenvironment complexity as well as a relevant physiological model. On the second hand, animal models are far from being able to predict human immune responses to drugs.
Here comes in the past decade of micro-engineering and biotechnology advances which has recently merged to create the so-called “organs-on-chip” technology. Though in vitro devices, this microfluidic-based technology tends to emulate the physiology of tissues, the complex biochemical microenvironment and the important mechanical constrains of cells.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Summary
The current drug development practice lacks reliable and sensitive techniques to evaluate the immunotoxicity of drug candidates, i.e., their effect on the human immune system. This, in part, has resulted in a high attrition rate for novel drug candidates. Organ-on-chip devices have emerged as key tools that permit the study of human physiology in controlled in vivo simulating environments. Furthermore, there has been a growing interest in developing the so-called “body-on-chip” devices to better predict the systemic effects of drug candidates. This review describes existing biomimetic immune organs-on-chip, highlights their physiological relevance to drug development and discovery, and emphasizes the need for developing comprehensive immune system-on-chip models. Such immune models can enhance the performance of novel drug candidates during clinical trials and contribute to reducing the high attrition rate as well as the high cost associated with drug development.
All the different immune cells originate from hematopoietic stem cells found in the bone marrow.
These hemocytoblasts then differentiate into either common myeloid progenitors or common lymphoid progenitors. The first myeloid cells will then give rise to various types of cells (neutrophils, basophils, macrophages…) which will scan tissues within the entire body for foreign antigens. The later (common lymphoid progenitors) will differentiate into lymphocytes. T and B lymphocytes both display receptors that allow them to be antigen-specific. These actors are major players in adaptive immunity due to their specific immunological memory features.
While giving a good summary of the origins of the different cell components of the immune system, this diagram does not reflect its complexity. Just as an example, we can mention the role of dendritic cells (originating from common myeloid progenitors) to activate T helper cells by first randomly phagocyting foreign cells, then relocating the antigen to their surface, and finally activate helper T cells. These TH cells once switched on can assist other white blood cells (maturation of B cells, activation of macrophages…).
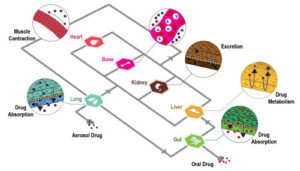
A higher level of complexity occurs when the entire immune homeostasis at the body level is required to ensure a proper immune response. Thus, in order to envisage physiologically relevant immune responses, an advanced in vitro system should represent each of the main immune organs: skin, gut, thymus, bone marrow, spleen, liver, and lymph node.
To date, three strategies have been followed to look at the poorly understood effects of drug candidates on the global immune system: in vitro, in vivo, and in silico studies.
Below is a table summarizing the different Organs-on-chip devices related to the immune system.
References
Shanti, A.; Teo, J.; Stefanini, C. In Vitro Immune Organs-on-Chip for Drug Development: A Review. Pharmaceutics 2018, 10, 278
The Organ Systems/Immune. (2017, July 23). Wikibooks, The Free Textbook Project. Retrieved 15:10, January 3, 2019 from https://en.wikibooks.org/w/index.php?title=The_Organ_Systems/Immune&oldid=3245932.