Evaporation as a major issue for long-term imaging
The majority of live-cell imaging experiments done by biologist require long-term analyses. One main issue that can appear is related to the evaporation of the medium. This is particularly true when the medium volume of the biological sample is very small (few micro-litters). Evaporation can be a critical factor and highly impacts on cells happiness if not viability.
As it is mentioned in Ettinger and colleagues,(1) the main way to avoid media evaporation and ensuing changes in osmotic pressure is to have sealed chambers. Cherry Biotech offer the possibility to work with close and sealed chambers as explained above.
Other proposals,(2,3) mention the possibility of having a sandwich of cover slips where the sample is in the middle so it is very difficult for the liquid to evaporate. The same research group mention the idea of using a seal chamber having the option of medium renewal.
Some other options are explained in the following article (4) but the majority of them present the same limitation. Both stage incubators or a thermalization chamber around the microscope (the two main systems allowing thermalization and humidity control) face the same constrain of not allowing a direct access to the sample.
Ultra fast temperature shift device for in vitro experiments under microscopy
Experimentation showing the efficiency of our microscopy chamber at avoiding evaporation
Our CherryTemp temperature controller is delivered with a set of sample mounting elements. Our R&D team spent energy to provide a simple, user-friendly and easy-to-mount chamber which prevents from any evaporation. We developed a test using the CherryTemp sample mounting system that is used for thermalization biological samples.
The objective of this test is to check the capability of our closed chamber to avoid evaporation over a long period of time.

1/ Methods

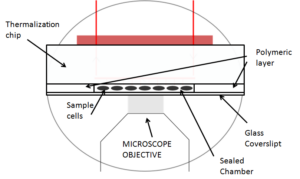
The sample mounting system provided with the CherryTemp system respects standards of microscopy: considering an inverted microscope set-up, it is composed of a cover slip, an approx 500µm thick, re-usable spacer which creates a sealed chamber when the thermalizing unit is finally placed on top.
Two sample mounting systems with two different spacers (A – white and B – transparent), both fully biocompatible, were used for this test. Spacer A is a standard silicon sheet commercially available whereas spacer B results from our developments. To mimic a biological sample medium and ease the visualisation, we used a coloured liquid. Finally, the three pieces (cover slip + spacer + thermalization unit) are properly maintained into an insert fitting standards of microscope stages.
2/ Results
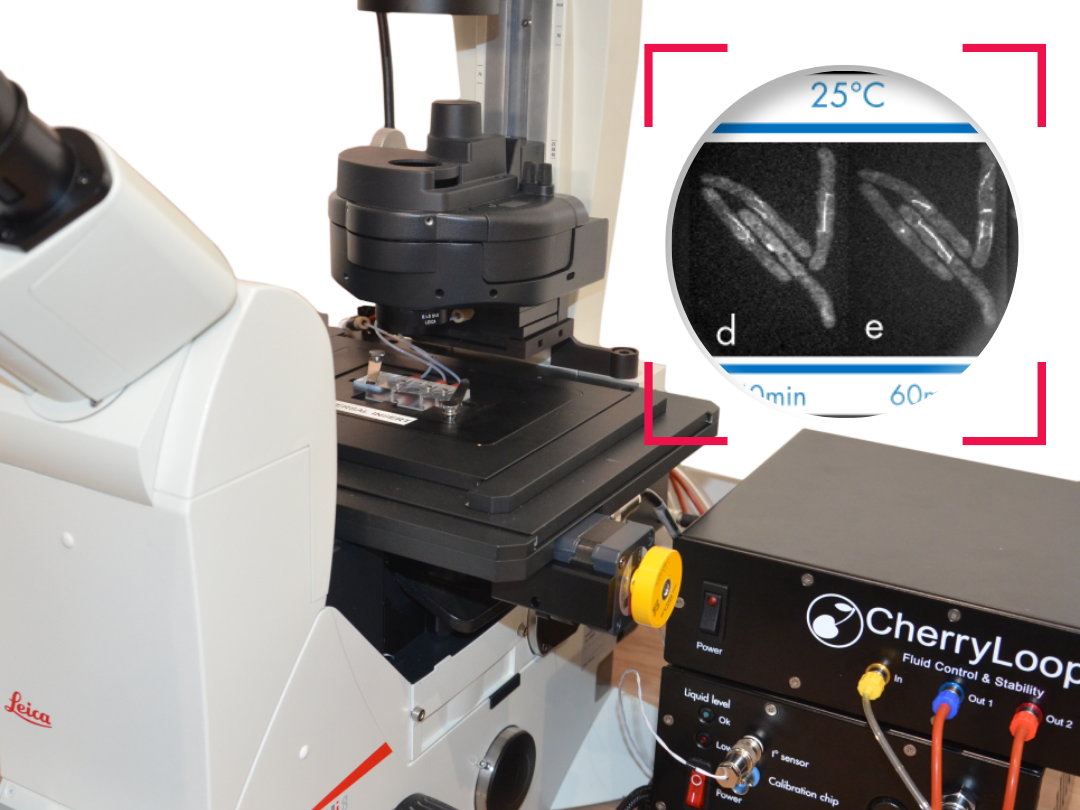
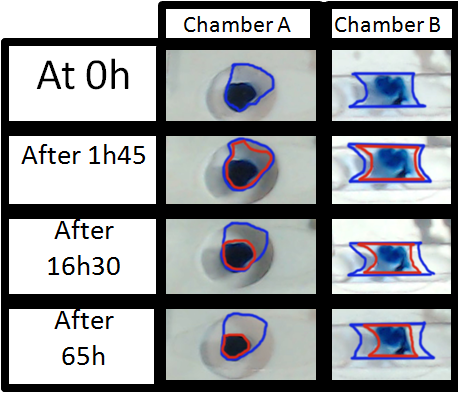
Figure 3 shows the evolution of the droplet after 1 h and 45 minute, then 16h and 30 minutes and finally after 65h. The main objective of these images is to make the evaporation process visual and easy to understand. The other main point is to show how important is to have a sealed chamber to keep in the medium and have no evaporation.
As it can be seen on the images the evolution of the medium inside chamber A and B is very different. Inside the blue circle is the initial medium inside each chamber.
- On chamber A the medium evaporates more than the 90% after the 16h. At 65h there is no medium available, just the colorant concentrated on a droplet
- The evolution in Chamber B is compatible with long-term imaging. Indeed, after 16h, the drop is though at the end of the test after 65 hours only half of the medium evaporated.
2/ Conclusion
This test shows the efficiency of this simple and user-friendly mounting system developed by the R&D department of Cherry Biotech.
The take-away observation is that spacer B provides a better sealing compared with the A one. This spacer B is the result of our focus to develop user-friendly devices. As mentioned by Ettinger, Dailey and Daum (1–3,5) perfect sealing is crucial to avoid all kind of evaporation as well as helping to keep the environment stable. The integration of this mounting system into our insert provides an ideal chamber to avoid evaporation while performing long-term imaging.
References
- Ettinger A, Wittmann T. Fluorescence live cell imaging. Methods Cell Biol [Internet]. 2014 [cited 2016 Oct 10];123:77–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24974023
- Dailey ME, Marrs GS, Kurpius D. Maintaining live cells and tissue slices in the imaging setup. Cold Spring Harb Protoc [Internet]. 2011 Apr [cited 2016 Oct 12];2011(4):pdb.top105. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21460059
- Daum JR, Potapova TA, Sivakumar S, Daniel JJ, Flynn JN, Rankin S, et al. Cohesion Fatigue Induces Chromatid Separation in Cells Delayed at Metaphase. Curr Biol [Internet]. 2011 Jun [cited 2016 Oct 11];21(12):1018–24. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0960982211005884
- Kulesa PM, Kasemeier-Kulesa JC. Construction of a Heated Incubation Chamber around a Microscope Stage for Time-Lapse Imaging: Figure 1. Cold Spring Harb Protoc [Internet]. 2007 Jul [cited 2016 Oct 12];2007(7):pdb.prot4792. Available from: http://www.cshprotocols.org/lookup/doi/10.1101/pdb.prot4792
- Sivakumar S, Daum JR, Gorbsky GJ. Live-Cell Fluorescence Imaging for Phenotypic Analysis of Mitosis. In 2014 [cited 2016 Oct 11]. p. 549–62. Available from: http://link.springer.com/10.1007/978-1-4939-0888-2_31
FAQ
Media evaporation is a main issue for long-term analyses. This problem is especially pronounced when the medium volume for the biological sample is very small, such as a few micro-litters. Evaporation is a serious factor. It can greatly affect cell happiness and, if not controlled, cell viability. The main method suggested to avoid medium evaporation is the use of sealed chambers. This method also prevents ensuing changes in osmotic pressure. Other proposals have mentioned using a sandwich of cover slips to hold the sample. Another mentioned option is a seal chamber that has the option for medium renewal.
Other systems exist that allow for thermalization and humidity control. The two main systems mentioned are stage incubators and thermalization chambers that are placed around the microscope. A limitation is associated with the majority of these systems. They often face the constraint of not permitting direct access to the sample being imaged. The main method for avoiding media evaporation and the resulting changes in osmotic pressure is to use sealed chambers. Some systems provide the option to work with these closed and sealed chambers. Other suggestions include using a sandwich of cover slips or a sealed chamber that allows for medium renewal.
A test was developed to check the capability of a closed chamber to avoid evaporation over a long period. The sample mounting system used in the test respects microscopy standards for an inverted setup. It is composed of three pieces: a cover slip, a spacer, and a thermalizing unit. The spacer is re-usable and approximately 500µm thick. The thermalizing unit is placed on top, which creates a sealed chamber. These parts are held in an insert. Two different, fully biocompatible spacers were tested: A and B. Spacer A was a standard, commercially available silicon sheet. Spacer B resulted from in-house developments. A coloured liquid was used to mimic the sample medium and for ease of visualisation.
The evolution of the medium inside chamber A and chamber B was observed to be very different. Images were taken over 65 hours to make the evaporation process visual. Inside chamber A, which used the standard silicon spacer, the medium evaporated by more than 90 percent after 16 hours and 30 minutes. After 65 hours, there was no medium available in chamber A, leaving just the concentrated colorant. The results for chamber B were different. The evolution in Chamber B was determined to be compatible with long-term imaging needs. After 16 hours, the drop was still present. At the end of the 65-hour test, only half of the medium in chamber B had evaporated. This showed that spacer B provides better sealing.