Introduction
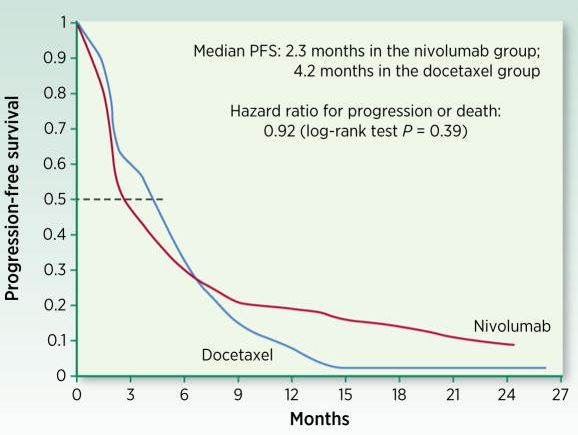
In this paper, Anagnostou and colleagues “discuss endpoints used in I-O trials to date and potential optimal endpoints for future early- and late-phase clinical development of I-O therapies”. They establish that classical dose-response patterns are often nonapplicable in the field of immuno-oncology, during the toxicity and response assessment phases in clinical trials. In this paper, they discussed new possible endpoints and methods to obtain clinically relevant data. This is particularly needed when addressing the ongoing development of checkpoint modulators.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Summary
Immuno-oncology (I-O) has required a shift in the established paradigm of toxicity and response assessment in clinical research. The design and interpretation of cancer clinical trials has been primarily driven by conventional toxicity and efficacy patterns observed with chemotherapy and targeted agents, which are insufficient to fully inform clinical trial design and guide therapeutic decisions in I-O. Responses to immune-targeted agents follow nonlinear dose-response and dose-toxicity kinetics mandating the development of novel response evaluation criteria. Biomarker-driven surrogate endpoints may better capture the mechanism of action and biological response to I-O agents and could be incorporated prospectively in early-phase I-O clinical trials. While overall survival remains the gold standard for evaluation of clinical efficacy of I-O agents in late-phase clinical trials, exploration of potential novel surrogate endpoints such as objective response rate and milestone survival is to be encouraged. Patient-reported outcomes should also be assessed to help redefine endpoints for I-O clinical trials and drive more efficient drug development. This paper discusses endpoints used in I-O trials to date and potential optimal endpoints for future early- and late-phase clinical development of I-O therapies.