Introduction
SARS-CoV-2 is the virus that caused the COVID-19 pandemic and its devastating effects on global health and economics. The virus’s transmission and pathology appear to be mediated through the respiratory system via interaction of the viral spike protein with the ACE-2 receptor, which is expressed differently on different cells in the respiratory system. ACE-2 expression has been reported on lung alveolar epithelial cells, enterocytes of the small intestine, circulatory endothelial cells, arterial smooth muscle cells, adipose tissue, bone marrow, duodenum, endometrium, heart, kidney, testis, and thyroid, implying that COVID-19 may have a direct effect on those tissues. Furthermore, COVID-19 infection has been linked to severe liver damage and abnormal liver function tests. Secondary effects of the cytokine cascade, hypoxia, underlying liver disease, or infection of ACE-2 positive cholangiocytes have all been linked to changes in liver function. There have also been reports of coronavirus particles in hepatocytes without a clear infection mechanism. This study highlights that in human primary hepatocytes, SARS-CoV-2 spike protein binding probably involved other receptors and mechanisms than only ACE-2.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract of Binding of the SARS-CoV-2 Spike Protein to the Asialoglycoprotein Receptor on Human Primary Hepatocytes and Immortalized Hepatocyte-Like Cells by Confocal Analysis.
The author talking about the background discusses, “The SARS-CoV-2 virus may have direct or indirect effects on other human organs beyond the respiratory system and including the liver, via binding of the spike protein. This study investigated the potential direct interactions with the liver by comparing the binding of SARS-CoV-2 spike proteins to human AT2-like cells, primary human hepatocytes and immortalized hepatocyte-like hybrid cells. Receptors with binding specificity for SARS-CoV-2 spike protein on AT2 cells and hepatocytes were identified.
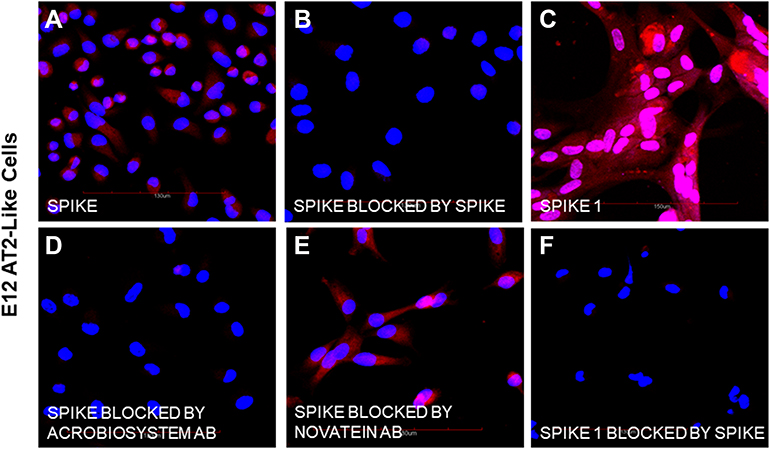
Methods used in the study: The specific binding of biotinylated spike and spike 1 proteins to undifferentiated human E12 MLPC (E12), E12 differentiated alveolar type 2 (AT2) cells, primary human hepatocytes (PHH) and E12 human hepatocyte-like hybrid cells (HLC) was studied by confocal microscopy. We investigated the expression of ACE-2, binding of biotinylated spike protein, biotinylated spike 1 and inhibition of binding by unlabeled spike protein, two neutralizing antibodies and an antibody directed against the hepatocyte asialoglycoprotein receptor 1 (ASGr1).
Results: E12 MLPC did not express ACE-2 and did not bind either of spike or spike 1 proteins. AT2-like cells expressed ACE-2 and bound both spike and spike 1. Both PHH and HLC did not express ACE-2 and did not bind spike 1 protein. However, both PHH and HLC actively bound the spike protein. Biotinylated spike protein binding was inhibited by unlabeled spike but not spike 1 protein on PHH and HLC. Two commercial neutralizing antibodies blocked the binding of the spike to PHH and HLC but only one blocked binding to AT2. An antibody to the hepatocyte ASGr1 blocked the binding of the spike protein to PHH and HLC.
Conclusion: The absence of ACE-2 receptors and inhibition of spike binding by an antibody to the ASGr1 on both PHH and HLC suggested that the spike protein interacts with the ASGr1. The differential antibody blocking of spike binding to AT2, PHH and HLC indicated that neutralizing activity of SARS-CoV-2 binding might involve additional mechanisms beyond RBD binding to ACE-2.
Keywords: asialoglycoprotein receptor, E12 MLPC, SARS-CoV-2, AT2, human hepatocytes, spike proteins.
References
Collins DP, Steer CJ. Binding of the SARS-CoV-2 Spike Protein to the Asialoglycoprotein Receptor on Human Primary Hepatocytes and Immortalized Hepatocyte-Like Cells by Confocal Analysis. Hepat Med. 2021;13:37-44
https://doi.org/10.2147/HMER.S301979