Introduction
Drosophila pupae have been used in research as a model to study developmental transitions like metamorphosis. The developmental transformations occurring from the 3rd instar larva till the adult fly formation have been studied in the fruit fly pupae , which are easy to manipulate, dissect and image.

Ultra fast temperature shift device for in vitro experiments under microscopy
Drosophila pupa as a model for developmental research
The pupal stage is a phase in the life cycle of the fruit fly which follows encapsulation of the 3d instar larva inside a pupal case or puparium. Pupal stage lasts around 4 or 5 days, although this time depends on the sex (sexual dimorphism: pupal stage is longer in males), circadian clocks [3] or temperature [4].
Metamorphosis and pupal eclosion
During pupal stage many larval structures are lysed and new structures are formed from the imaginal disks. Imaginal disks are developed from larval undifferentiated cells and give rise to multiple adult structures such as the adult head, legs, wings, thorax and reproductive apparatus. Some larval structures like the nervous system or gonads are preserved during the pupal stage [1]
Eclosion is the process by which an adult fly emerges from the pupal case. In this video can be observed drosophila pupae eclosion. As it can be observed in the video, the puparium turns black before eclosion. Eclosion rate and pupa lethality are two parameters often used as readouts for developmental defects in genetic screens.
Metamorphosis and pupal eclosion are highly energy-demanding processes. Prepupal and late pupal (right before eclosion) steps have high energetic rates. These patterns follow metabolic activity of pupal tissues, which becomes very low after larval tissue lysis and increases again during adult tissue differentiation. These metabolic rates are also temperature dependent (becoming higher at high temperatures) [4].
Gene expression changes drive the metamorphosis process, with the steroid hormone ecdysone playing a central role, together with other hormones and neuropeptides [2]. Ecdysone is first synthesized in the larval prothoracic gland and plays also a role in the moulting of larval stages. Pulses of ecdysone during the pupal stage allow for imaginal disc differentiation and adult structure formation through different transcription factors.
Muscle morphogenesis in fruit fly pupae
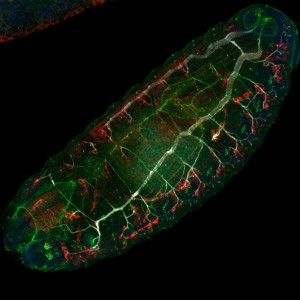
Drosophila is a powerful model system to study muscle morphogenesis throughout its life cycle, from embryo to adult. After puparium formation, major developmental steps occur in muscle morphogenesis. Larval muscles are lysed in the pupal stage. Additionally, undifferentiated myoblasts AMP (adult muscle precursors) present in the larva get fused and go through differentiation into FC (founder cell myoblasts) or FCM (fusion competent myoblasts). Indirect flight muscles (IFM), leg muscles and abdominal muscles are formed during the pupal stage [5]. Imaging of both fixed and live muscle upon pupae dissection have been key tools to understand muscle morphogenesis.

Dissection and mounting protocols for fly pupae
Mouning pupae protocols for whole body imaging or pupae dissection for observation of live tissues have been extensively used in developmental biology. Here are some examples of protocols to dissect or mount pupae for live cell imaging. Removing of the opaque pupal case is a first step common in all protocols.
General protocol for pupa dissection
Other protocols for pupa dissection can be found for: muscle observation [5], pupal eye discs [6], abdominal development [7], pupal wings [8].
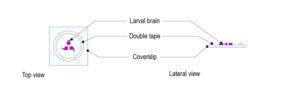
Protocol for mounting pupa compatible with temperature control with CherryTemp
References
- [1] Hales, KG et al. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics.(2015) https://www.ncbi.nlm.nih.gov/pubmed/26564900
- [2] Yamanaka N. et al. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol (2013)
- https://www.ncbi.nlm.nih.gov/pubmed/23072462
- [3] Qiu J. and Hardin P. Developmental state and the circadian clock interact to influence the timing of eclosion in Drosophila melanogaster. J Biol Rhythms.(1996) https://www.ncbi.nlm.nih.gov/pubmed/8695895
- [4] Merkey AB et al. Energetics of metamorphosis in Drosophila melanogaster. J. Insect. Physiol (2011) https://www.ncbi.nlm.nih.gov/pubmed/21810426
- [5]Weitkunat M. A guide to study Drosophila muscle biology. Methods (2014)
- [6] Tea JS et al. Dissection and mounting of Drosophila pupal eye discs. J Vis Exp (2014)
- https://www.ncbi.nlm.nih.gov/pubmed/25406645
- [7] Ninov N. Live imaging of epidermal morphogenesis during the development of the adult abdominal epidermis of Drosophila. Nature Protocols 2007 https://www.ncbi.nlm.nih.gov/pubmed/18079706
- [8] Bolatto C. et al. A Rapid and Efficient Method to Dissect Pupal Wings of Drosophila Suitable for Immunodetections or PCR Assays. J Vis Exp 2017 https://www.ncbi.nlm.nih.gov/pubmed/29364201