Scaling up organs on a chip to get a functional body on a chip
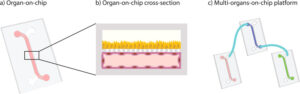
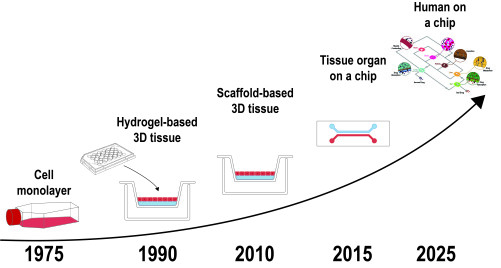
Recent innovations in microfluidic technologies have produced organ on a chip (OoC) platforms, which integrate advanced 3D tissue-engineered constructs with microfluidic networks to minimize the shortcomings of in vitro 2D models. Such a cohesive platform enables important physiological cues, such as the vasculature and interstitial fluid flow, which improves the emulation of the in vivo physiological conditions for studying stem cell differentiation, metastasis, and so on. In addition, interspecies differences can be eliminated through the use of human cells. Furthermore, researchers have begun to investigate the interconnection of multiple OoC systems into a single network, in order to emulate inter-organ relationships and ultimately develop human-body-like microphysiological systems. The perspectives opened by this new in vitro tool take place in the fields of personalized medicine, pre-clinical trials, toxicology, and drug development.
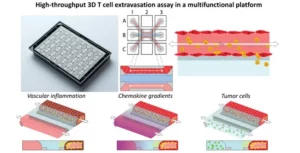
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
What are the challenges to tackle to get a functional body on-chip?
To go from a single organ-on-a-chip to a body on a chip, scientists have to solve issues. The first one is the heterogeneity between all the different organ culture conditions. Up to date, and since organs-on-chips are developed individually, researchers are both focusing on the finding of the optimal conditions allowing the maintenance of one single organ (e.g. mechanical constraints, three-dimensional architecture, the composition of the medium…) or allowing to perform in vitro tests. Building a body-on-chip means to both find a common medium composition for all the organs and to respect each specific and required organ environment. The second one is linked to the vasculature itself. Indeed, in the human body, organs are interconnected by blood vessels responsible for the maintenance of blood pressure with the help of the heart. To be as close as possible to human physiology, the device will have to possess its own vasculature, meaning that each microfluidic channel has to mimic a vessel. In addition, the medium flow will have to be “pulsatile” to mimic the heart action. Some solutions have already been proposed (Chen Y et al, 2017, Lab Chip). One other challenge consists of including the immune system into the micro-system. The main issue comes from the function of the immune cells themselves. Indeed, they are made to “attack” everything which is not related to itself. It means that a chip containing an immune system has to come from the same donor to avoid any “self-destruction” of the organs. The most sophisticated system might be an autonomous system in terms of self-generation of blood cells by the bone marrow and a contractile “mini heart” to generate the blood flow.
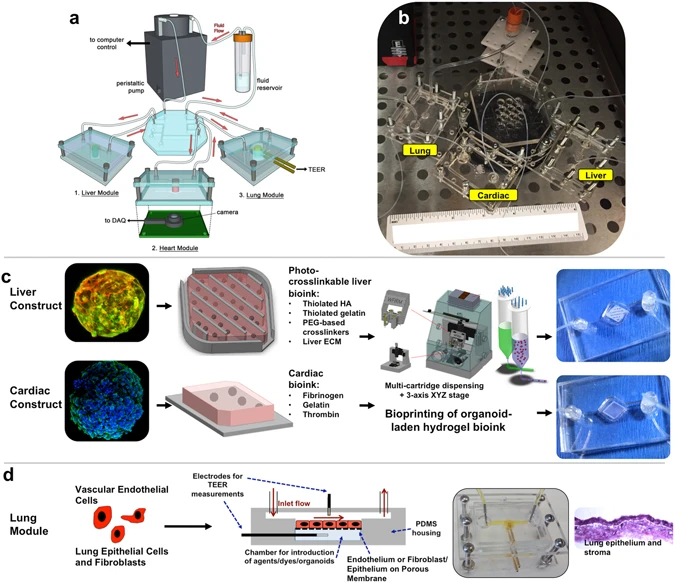
Main features of such an integrated into vitro system
Despite this complexity, a body-on-chip brings highly valuable new features, notably in the field of personalized medicine. Indeed, coupled with the most recent progress in cell culture (IPS cells, 3D printed organs…), they will allow the culture of different organs from one single patient. Thus, the efficiency of a particular pharmaceutical or treatment procedure can be measured at the whole-body level, and side effects can be easily spotted on each organ. In addition, target organs for a specific chemical (or a mixture of chemicals) can be easily identified. The medium collection and direct organ observation will characterize the effects of the considered chemical. Moreover, the design of the chip will easily take into account various routes of exposure and their differential effects on each organ. This is really important in the toxicology and risk assessment fields. For example, in the case of an oral administration of a chemical, the first organ in contact will be the liver, which will metabolize it and release many metabolites (either active or not) in the medium flow, whereas an intravenous administration will skip this step and will equally distribute the chemical to all organs.
References
Nath, G.R. Devi / Pharmacology & Therapeutics 163 (2016) 94–108
See also :
Drug testing could get a boost from MIT’s ‘body on chip’
Scientists develop ‘body-on- a-chip’ system to accelerate testing of new drugs
Human Organs-on-Chips from Harvard Wyss institute