Introduction
Non-adherent cells are difficult to image because of movement. This is the case of living cells which are motile, but also of fixed cells which will move due to Brownian motion. While doing fluorescent microscopy, it is critical to prevent movement of cells to be imaged. This is particularly important when doing multichannel fluorescent microscopy, when there is a need to merge the two channels for colour overlays. There are several methods to prevent cell movement during cell imaging, and this Scientific Note focuses on agar pads.

Ultra fast temperature shift device for in vitro experiments under microscopy
What is an agar pad?
Agar pads prevent both motility of live cells and Brownian motion of fixed cells. This makes them the method of choice for live imaging of multiple model organisms: bacteria, yeast, worm embryos and adult worms, plant, zebrafish embryos. They can be coupled to additional methods to further enhance immobilization: for instance, the use of anesthetics for C. elegans.
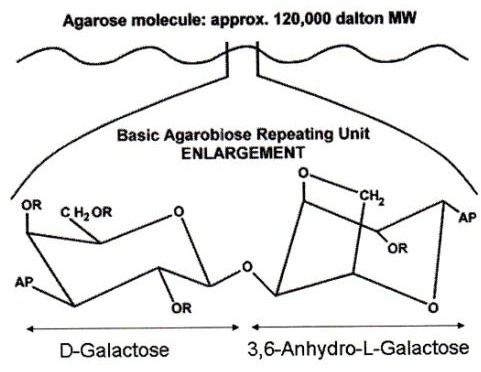
What is an agar pad? Although they are commonly referred as such, they are commonly made of agarose. Agar is a polysaccharide obtained from red algae, while agarose is the main component of agar, obtained after agar purification. Therefore, they are thin flat layers of an agarose matrix (see image). As a polymer, agarose creates a porous matrix where cells (or molecules for molecular biology applications) are trapped. However, nutrient and gas exchange occurs through the matrix making this method compatible with live imaging. No toxicity for the cells due to growth in agar has been described.
Agarose percentage to make pads can vary. Very standard percentages are from 0.5% to 2%. Higher percentages of agarose (up to 10%) have been used to immobilize adult worms, with the advantage of the lack of toxicity compared to other methods. Adult worms in agarose pads can be imaged for hours if the pad is properly sealed (avoiding desiccation) [3]. Replacement of agarose by other polysaccharides is as well possible. For instance, while 2% agar is the standard pad used for C. elegans embryo imaging, this can be replaced for 4% sucrose 0.1M NaCl for delicate early embryos or embryos sensitive to osmotic stress (Hyman Lab protocol, [2])
Melting and gelling temperatures depend on the type of agarose. Standard agarose is liquid after dissolution in water at high temperatures and solidifies around 35-45°C (depending on the concentration). Low melting agaroses remain fluid at much low temperatures (30°C).
What is an agar pad?
Agarose pads must be prepared in a way to ensure they are thin and flat, so they can be placed in a sandwich between a glass slide and a coverslip. Thickness of the pad will depend on the organism to be imaged. The following protocol shows how to prepare an 250uM thick pad by using simple and non-expensive laboratory materials: glass slides and laboratory tape. Note that thickness of the tape is a critical factor. If the tape is too thin, this will lead to very thin agar pads which will be very fragile and difficult to manipulate. (see our Agarose pad method for C. elegans temperature-sensitive embryos).
This mounting protocol is compatible as well with thicker agar pads. This could be needed if thicker specimens need to be imaged, like zebrafish embryos. The following video shows how an 1mm thick pad (percentage of agar 2%) can be thermalized using a CherryTemp device. The thickness of the agar slows down thermal transfer through the pad, although a homogeneous temperature across the pad is achieved after 30 seconds.The main limitations of agar pads for live cell imaging are the impossibility to change media (for instance to apply drugs), the risk of pad drying, and the limiting nutrient availability for long term imaging experiments. Microfluidic technologies, which ensure a more controlled environment for the cells, aim to overcome these limitations [4].
The main limitations of agar pads for live cell imaging are the impossibility to change media (for instance to apply drugs), the risk of pad drying, and the limiting nutrient availability for long term imaging experiments. Microfluidic technologies, which ensure a more controlled environment for the cells, aim to overcome these limitations [4].
References
[1] Agarose Wikipedia page: https://en.wikipedia.org/wiki/Agarose
[2] Protocol from Hyman Lab (MPI): Resources, Methods: https://hymanlab.mpi-cbg.de/hyman_lab/c-elegans/
[3] Kim E.et al. Long-term imaging of Caenorhabditis elegans using nanoparticle-mediated immobilization. PLoS One. (2013). https://www.ncbi.nlm.nih.gov/pubmed/23301069
[4] Ducret A. et al. A microscope automated fluidic system to study bacterial processes in real time. PLoS One. (2009). https://www.ncbi.nlm.nih.gov/pubmed/19789641