Introduction
Adipose Tissue is very versatile and essential for studies related to Obesity and Diabetes, however, the high buoyancy, fragility, and heterogeneity of primary adipocytes have largely prevented their use in drug discovery efforts, emphasizing the importance of human stem cell-based approaches.
Adipose Tissue Microphysiological systems can provide us with robust models for drug discovery as new differentiation conditions yielding hormonally responsive iADIPO are derived using three separate insulin sensitivity assays, namely glucose and fatty acid absorption and inhibition of lipolysis, as functional readouts.
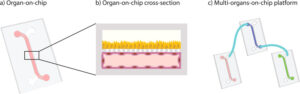
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The authors state that “Impaired white adipose tissue (WAT) function has been recognized as a critical early event in obesity-driven disorders, but high buoyancy, fragility, and heterogeneity of primary adipocytes have largely prevented their use in drug discovery efforts highlighting the need for human stem cell-based approaches.
Here, human stem cells are utilized to derive metabolically functional 3D adipose tissue (iADIPO) in a microphysiological system (MPS). Surprisingly, previously reported WAT differentiation approaches to create insulin-resistant WAT are ill-suited for type-2 diabetes mellitus drug discovery.
Using three independent insulin sensitivity assays, i.e., glucose and fatty acid uptake and suppression of lipolysis, as the functional readouts new differentiation conditions yielding hormonally responsive iADIPO are derived.
Through concomitant optimization of an iADIPO-MPS, it is able to obtain WAT with more unilocular and significantly larger (≈40%) lipid droplets compared to iADIPO in 2D culture, increased insulin responsiveness of glucose uptake (≈2-3 fold), fatty acid uptake (≈3-6 fold), and ≈40% suppressing of stimulated lipolysis giving a dynamic range that is competent to current in vivo and ex vivo models, allowing to identify both insulin sensitizers and desensitizers.”.
References
Qi L, Zushin PH, Chang CF, Lee YT, Alba DL, Koliwad SK, Stahl A. Probing Insulin Sensitivity with Metabolically Competent Human Stem Cell-Derived White Adipose Tissue Microphysiological Systems. Small. 2021 Nov 10:e2103157. doi: 10.1002/smll.202103157. Epub ahead of print. PMID: 34761526.
FAQ
Adipose tissue is considered necessary for studies related to obesity and diabetes. The use of primary adipocytes in drug discovery has been largely prevented by several challenges. These cells have high buoyancy, are fragile, and show a great degree of heterogeneity. These properties have made their application difficult. Impaired function in white adipose tissue (WAT) is also seen as a critical early event in disorders driven by obesity. This situation highlights the need for methods that are based on human stem cells. Adipose tissue microphysiological systems can provide suitable models for drug discovery.
It was found that previously reported differentiation approaches for white adipose tissue (WAT) were not well-suited for drug discovery related to type-2 diabetes mellitus. This was true even for methods intended to create insulin-resistant WAT. This created a need for new differentiation conditions. These new conditions were derived to produce iADIPO (adipose tissue from stem cells) that is responsive to hormones. The development process used three independent assays for insulin sensitivity. These assays served as the measurements of metabolic activity. The specific assays were glucose uptake, fatty acid uptake, and the suppression of lipolysis.
An iADIPO-MPS (microphysiological system) was concomitantly refined. This process resulted in white adipose tissue (WAT) with different characteristics compared to iADIPO in 2D culture. The WAT within the microphysiological system was observed to have more unilocular lipid droplets. These droplets were also considerably larger, by approximately 40 percent. An increased insulin responsiveness was also seen in the 3D model. Glucose uptake responsiveness was two to three times greater. Fatty acid uptake responsiveness was three to six times greater. Additionally, the system demonstrated an approximately 40 percent suppression of stimulated lipolysis.
The refined iADIPO-MPS provides a range of responses. This range is comparable to current in vivo and ex vivo models. This level of operation is important for research. It permits the identification of both insulin sensitizers and desensitizers. This was a drawback of earlier WAT differentiation methods, which were found to be unsuitable for type-2 diabetes drug discovery. The new system uses human stem cells. These cells are used to derive metabolically operative 3D adipose tissue within a microphysiological system. The model’s performance is verified using three separate assays for insulin sensitivity.