Adiponectin and Diabetes
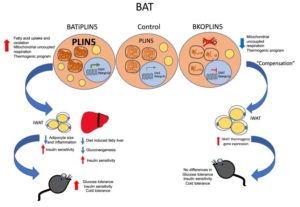
One-third of Americans are expected to have diabetes by 2050. Understanding how to avoid insulin resistance will be helpful. Adiponectin has been shown to play a significant role in improving insulin sensitivity, but the processes of how adiponectin enhances insulin sensitivity are uncertain. Adiponectin, a hormone produced in adipose tissue, interferes with blood sugar control and may provide a beneficial therapeutic lead for type 2 diabetes because it increases insulin sensitivity and stabilizes blood sugar levels. However, how this occurs is unknown. Li et al. observed that in mice suffering from insulin resistance, adiponectin therapy decreased the amount of triglyceride in the liver and in the skeletal muscles. It achieved so by increasing lipid secretions from skeletal muscle and increased fatty acid oxidation in the adipose tissue. The results triggered a decline in the levels of diacylglycerol in the membranes of the liver and muscle cells, thereby reducing the function of protein kinase C family members that disrupt insulin signaling.

How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Animal testing and Adiponectin
Adiponectin seems to be a promising treatment choice for type 2 diabetes, but how it impacts insulin tolerance remains uncertain. Two weeks of globular adiponectin (gAcrp30) therapy decreased fasting plasma glucose, triglyceride (TAG), and insulin concentrations and reversed whole-body insulin resistance, which may be due to changes in both insulin-mediated repressions of endogenous glucose production and in insulin-stimulated glucose absorption in muscle and adipose tissues. These increases in liver and muscle sensitivity were associated with approximately 50% decreases in liver and muscle triglyceride content, which occurred irrespective of reductions in overall ceramide content. Reductions in plasma PM DAG levels were associated with reduced PKCε translocation in the liver and skeletal muscle, which resulted in PKCθ and PKCε translocation, decreased insulin-stimulated PI3K activity, and decreased Akt phosphorylation. Both G protein-coupled receptor 30, and full-length adiponectin activated the cAMP/protein kinase A cascade, as well as muscle fatty acid oxidation. gAcrp30 and Acrp30 injections improved TAG absorption in eWAT and may have been due to increased LPL activity. These results indicate that adiponectin and adiponectin associated molecules reverse lipid-induced liver and muscle insulin resistance by decreasing ectopic lipid storage in these tissues, resulting in reduced plasma membrane sn-1,2-DAG-induced PKA activity and increased insulin signaling. Adiponectin is also responsible for stimulating the accumulation of TAG in the eWAT and muscle, but also for stimulating the use of fat for energy output in the muscles.
Type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD) are among the leading causes of morbidity and mortality in the adult population worldwide and are correlated with disease in multiple organ systems, including atherosclerotic vascular disease. (ASCVD). Insulin resistance is a key factor in the progression of type 2 diabetes and metabolic syndrome. Adiponectin, an adipokine, is associated with many disorders including anti-diabetic, anti-inflammatory, and anti-atherosclerotic effects. Plasma adiponectin levels are inversely associated with obesity and insulin resistance and have reduced in T2DM. Adiponectin is found in humans in its maximum length and in its C-terminal globular (AKAP30) shape (gAcrp30). The C-terminal globular fragment is formed by proteolytic cleavage and a biologically active molecule is produced. Adiponectin plays a pivotal function in insulin resistance, but the mechanism by which it functions and affects insulin resistance is not well known.
A number of possible mechanisms for adiponectin’s glucose-lowering and insulin-sensitizing properties have been extracted mainly from in vitro and ex vivo research, including inhibition of gluconeogenesis, enhanced fatty acid oxidation in liver and muscle, and decreased ceramide levels in the liver by activation of the hepatic ceramidase enzyme. Further studies about the use of adiponectin as a potential target for therapy for Type 2 Diabetes in humans will be needed. Replicate such promising results using an in vitro 3D Human Vascularized 3D Adipose Tissue in a Microphysiological Systems (such as Cubix) will have the potential to strongly valorize such innovative findings on adiponectin.