Introduction
The supporting vasculature is critical to the development of Adipose Tissue (AT) engineered models. Although it is essential for AT function and long-term maintenance, the Adipocyte-Endothelium Crosstalk is poorly understood.
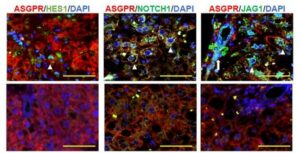
Because the crosstalk encompasses physiological adaptations such as blood flow alterations and molecular changes, the research relies largely on integrated models. In accordance with this, determining the precise function of Extracellular Vesicles in White Adipose Tissue biological control necessitates the development of novel experimental techniques as well as integrated models. Correlation studies are frequently used to infer the function of Extracellular Vesicles, while methods that inhibit exosome signaling in a cell-specific way are currently unavailable. Learn more through this review below by Sabaratnam et al.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract of Adipocyte-Endothelium Crosstalk in Obesity
The authors state that “Obesity is characterized by pathological adipose tissue (AT) expansion. While healthy AT expansion enhances systemic insulin sensitivity, unhealthy AT expansion through increased adipocyte size is associated with insulin resistance, fibrosis, hypoxia, and reduced adipose-derived adiponectin secretion. The mechanisms causing the unhealthy AT expansion are not fully elucidated; yet, dysregulated crosstalk between cells within the AT is an important contributor.
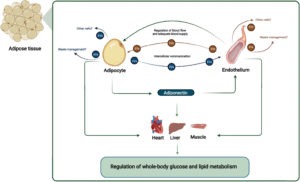
Evidence from animal and human studies suggests a crucial role of the crosstalk between vascular endothelium (the innermost cell type in blood vessels) and adipocytes for metabolic homeostasis. Arterial endothelial cells are directly involved in maintaining normal organ functions through local blood flow regulation. The endothelial-dependent regulation of blood flow in AT is hampered in obesity, which negatively affects the adipocyte. Moreover, endothelial cells secrete Extracellular Vesicles (EVs) that target adipocytes in vivo.
The endothelial EVs secretion is hampered in obesity and may be affected by the adipocyte-derived adipokine adiponectin. Adiponectin targets the vascular endothelium, eliciting organ-protective functions through binding to T-cadherin. The reduced obesity-induced adiponectin binding of T-cadherin reduces endothelial EV secretion. This affects endothelial health and cell-cell communication between AT cells and distant organs, influencing systemic energy homeostasis.
This review focuses on the current understanding of endothelial and adipocyte crosstalk. We will discuss how obesity changes the AT environment and how these changes contribute to obesity-associated metabolic disease in humans. Particularly, we will describe and discuss the EV-dependent communication and regulation between adipocytes, adiponectin, and the endothelial cells regulating systemic energy homeostasis in health and metabolic disease in humans.”
References
Sabaratnam R, Svenningsen P. Adipocyte-Endothelium Crosstalk in Obesity. Front Endocrinol (Lausanne). 2021 Aug 12;12:681290. doi: 10.3389/fendo.2021.681290. PMID: 34456860; PMCID: PMC8387580.
FAQ
Obesity is characterised by a pathological expansion of adipose tissue (AT). This unhealthy expansion is linked to an increase in the size of adipocytes, or fat cells. It is associated with several negative conditions. These include insulin resistance, fibrosis, and hypoxia. A reduction in adiponectin secretion is also observed. In contrast, healthy AT expansion is different. It is known to enhance the body’s systemic insulin sensitivity. The mechanisms that cause unhealthy expansion are not completely understood. It is known, however, that dysregulated communication, or crosstalk, between the cells within the AT is an important contributing factor.
Adipocyte-endothelium crosstalk is the bidirectional interaction between adipocytes and the vascular endothelium, which is the innermost cell type in blood vessels. This communication is essential for metabolic homeostasis. It is also necessary for adipose tissue function and its long-term maintenance. The process involves complex physiological adaptations, such as molecular changes and alterations in blood flow. This complexity is why the crosstalk is still poorly understood. Research must rely on integrated models. A specific challenge is studying Extracellular Vesicles (EVs). New experimental techniques are needed to determine their precise function. Methods to inhibit EV signaling in a cell-specific way are not currently available.
The interaction between the endothelium and adipocytes is an essential part of metabolic health. Arterial endothelial cells help maintain organ functions by regulating local blood flow. This endothelial-dependent regulation of blood flow within adipose tissue is hampered in obesity. This disruption has a negative effect on the adipocytes. Endothelial cells also secrete Extracellular Vesicles (EVs) that target adipocytes. This secretion of EVs is also hampered in obesity. These issues are part of the dysregulated crosstalk seen in unhealthy adipose tissue. This altered environment is a key contributor to obesity-associated metabolic disease.
Adiponectin is an adipokine, a substance derived from adipocytes. It targets the vascular endothelium. It performs organ-protective functions by binding to T-cadherin. Endothelial cells, in turn, secrete Extracellular Vesicles (EVs) that target adipocytes. The secretion of these EVs may be affected by adiponectin. In obesity, the binding of adiponectin to T-cadherin is reduced. This reduction leads to decreased endothelial EV secretion. This cascade negatively affects endothelial health. It also disrupts cell-cell communication within the adipose tissue and with distant organs. This ultimately influences the body’s systemic energy homeostasis.