Introduction
Animal models and two-dimensional (2D) cell cultures frequently fail to replicate human drug metabolism and liver toxicity in vivo, therefore there is a need for 3D Culture of HepaRG cells. Indeed, there is only a 30 to 50% concordance between animal and human models when it comes to hepatic drug toxicity. Furthermore, primary hepatocytes cultivated in 2D lose their functions quickly and cannot be used to examine pharmacological effects over time.
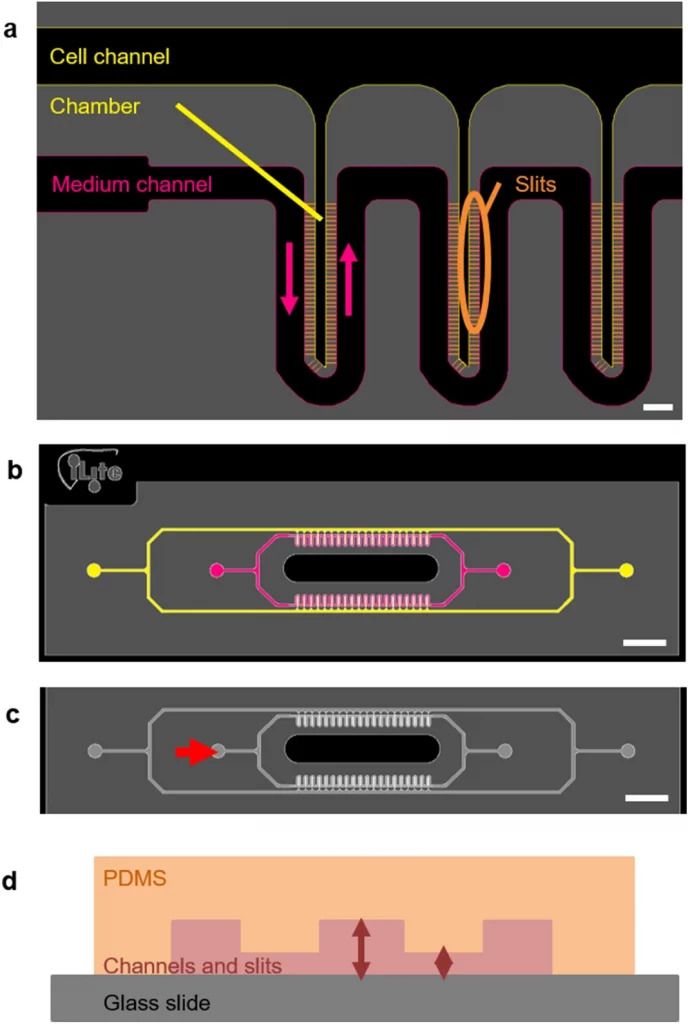
Their microfluidic chip was created to resemble the microstructure of the liver and to allow for more physiological culture conditions than earlier devices. Indeed, in addition to attaining 3D hepatocyte culture, the cells’ architecture allowed them to spatially organize into hepatocyte cords with in vivo-like dimensions.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The authors state that “The development of livers-on-a-chip aims to provide pharmaceutical companies with reliable systems to perform drug screening and toxicological studies. To that end, microfluidic systems are engineered to mimic the functions and architecture of this organ.
In this context we have designed a device that reproduces a series of liver microarchitectures, each permitting the 3D culture of hepatocytes by confining them to a chamber that is separated from the medium conveying channel by very thin slits.
We modified the structure to ensure its compatibility with the culture of hepatocytes from different sources. Our device was adapted to the migratory and adhesion properties of the human HepaRG cell line at various stages of differentiation. Using this device, it was possible to keep the cells alive for more than 14 days, during which they achieved a 3D organization and acquired or maintained their differentiation into hepatocytes.
Albumin secretion, as well as functional bile canaliculi, were confirmed on the liver-on-a-chip. Finally, an acetaminophen toxicological assay was performed. With its multiple micro-chambers for hepatocyte culture, this microfluidic device architecture offers a promising opportunity to provide new tools for drug screening applications.”
References
Boul M, Benzoubir N, Messina A, Ghasemi R, Mosbah IB, Duclos-Vallée JC, Dubart-Kupperschmitt A, Le Pioufle B. A versatile microfluidic tool for the 3D culture of HepaRG cells seeded at various stages of differentiation. Sci Rep. 2021 Jul 7;11(1):14075. doi: 10.1038/s41598-021-92011-7. PMID: 34234159; PMCID: PMC8263583.