Introduction
Once it is clear how to deal with 3D cell cultures it is time to focus on one type of cell and analyze its strengths and its limitations. In this case, liver cells will be analyzed.
There are some research groups trying to create liver-on-chip but they do not yet approach a complete solution in terms of cell-type diversity and complexity. [1-2] In order to better understand why it is necessary to get aware of the complexity of working with liver cells.
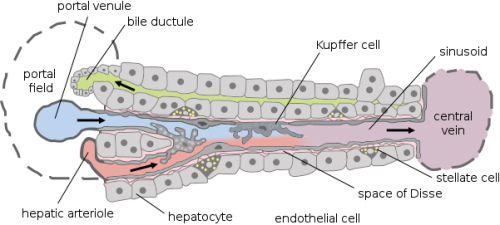
First, it is mandatory to know how the liver works and why it has a very complex structure. As can be seen in Figure 1, there are different types of cells such as hepatocyte, endothelial cells, Kupffer cells, or stellate cells. Even if hepatocytes are the main type of liver cell (in terms of number), it will be an error to forget the other types when creating a liver on a chip.
It is important to take care of the specific needs and functions of each of those cells and to know how they interact with each other because a more complex culture cell system can be recreated. Although, the hepatocytes required a very specific 3D structure to be able to keep their liver functions. This needs to be accomplished before any device is done because being able to have a liver functional unit is the key to long-term tests.
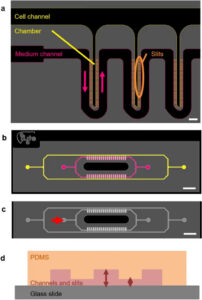
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
3D Liver Cell Cultures: building and growth
Depending on the source of the cells the culture technique will be different. This decision should be taken based on the final goal of the cell culture because the whole culture technique could be different.
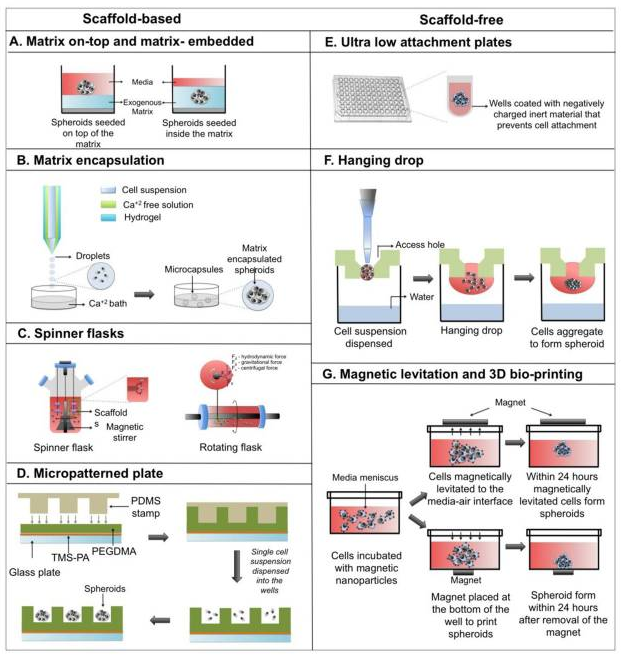
One source is the mammalian liver cells that are commonly used for toxicity tests or for biotransformation. These cells can grow by using the following technique (Figure 2) which consists of making the cells grow between two extracellular matrixes made of collagen. This is a well-known technique that can be considered as an intermediate between 2D and 3D cultures.
It is also possible to use bioreactors to grow the cells. There are many (microfluidic) devices on the market that are usable. [3-5] The advantage of using bioreactors for 3D cell culture is that they provide a continuous mass exchange of the medium and the option of decentralizing the oxygen supply. The limitations are related to a homogeneous mass exchange and with the size of the cell colonies. If there is not a good equal distribution of nutrients and oxygen many of the cells inside the bioreactor could die because of the lack of those compounds. The cell colony size is also a limitation because they should grow until a point where they consume the number of nutrients that is supposed for them and not more. If the growth is not controlled the homogeneity of the whole culture could be disrupted.
In order to build a 3D structure, cells can aggregate in the culture medium forming a scaffold system. This helps to keep the environment and allows good communication between the different cells of the 3D liver cell culture. However, a self-aggregate structure does not reflect the physiological reality.
The last novel technique that has been used more and more in the last few years is a culture process called Multicellular spheroid culture. It is based on the property of self-assembly of hepatocytes and other types of cells into aggregates. This way of culture can be used for different applications because it only needs to control one parameter: the size of the spheroid. (Figure 3). Indeed, if the spheroid is too big, the inner cells could not receive enough nutrients and oxygen and they will enter a necrotic state that will finally kill all the cells by dissemination of necrotic messenger. The key here is to control and maintain the growth rate under that critical point.
3D Liver Cell Culture: applications
The first well-known application is toxicity tests. The good thing about these 3D liver cell cultures is that they can be cultivated for a longer period than traditional 2D culture without trypsinization. Some groups have already proposed using the liver cell spheroids for toxicity test thanks to their stability and the fact that, thanks to a bigger surface area than in monolayer culture, molecules can interact easily compared with other cell cultures technique. [6-7]. Furthermore, cells are exposed to the chemical following a gradient of concentration: the outer cells are directly exposed, whereas the inner cells are exposed due to the diffusion of the molecule. This is more physiologically relevant than a 2D monolayer in which all the cells are equally exposed.
Another area of use of this culture type is the test of drug metabolism and pharmacokinetics. Because these kinds of cultures are closer to the in vivo conditions than 2D monolayer the extrapolation of data to humans is easier. This is presuming to reduce the time needed for the drug testing steps because of this “in vitro to human” extrapolation facilitation. [8-9]
3D liver cell culture and liver-on-chip: Limitations and challenges
The first limitation concerns the miniaturization of the bioreactor. Scaling down such devices is a very hard task because it is necessary to keep the critical cell mass. If the size is reduced this mass must be considered because even if the cell sample is small, it is necessary to have a fixed minimum number of cells to make the system reliable and functional. Another limitation is that reducing the environment size will make the nutrient and oxygenation process more complex because it will be harder to reach all the cells if space goes down. Furthermore, because of the small-medium volume, it will be impoverished quicker
It is important to mention that microdevices must assure to be closed systems if they want to compete with actual bigger bioreactors. The feasibility of such a feature needs to be addressed while moving from millimeters to microns’ scale.
The main challenge for microfluidic devices (applied to liver cell culture) is related to the complexity of the liver itself (different cell types and specific architecture). This means that inside the liver, and depending on the location, different cell types are encountered. It implies that we need to pay attention to the design of the device (close system, specific design…), the conception remaining closely linked to the aim of the study, and the addressed questions.
In conclusion, it is important to identify which tests need to be done with 3D cell culture, and think about the whole process.
3D liver cell culture in the Organ-on-chip context
A lot of researchers are studying the liver because it plays a major role in metabolizing a lot of drugs and compounds. There a need to develop new tools to make toxicity/pharmacokinetic tests faster, with a small sample but with high accuracy and more physiologically relevant. This is the main reason for scientists and industrial partners to be in the organ-on-chip race: being able to provide edge technology and bringing technical improvement for both health and medical safety.
References
- Toh Y-C, Lim TC, Tai D, Xiao G, van Noort D, Yu H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip [Internet]. 2009;9(14):2026.
- An Introduction to 3D Cell Culture Tools and Techniques. White Pap. 2012;(Figure 1):1–11.
- Mk B, Wang N, Lau W, Liu Q. Dynamic Culture Using Novel Slow Perfusion Biroeactor and Porous Polycaprolactone ( PCL ) Scaffolds for Bone Regeneration and Repair. Technology. :8902.
- 3D Biotek Bioreactor System – 3D Bioreactors | Sigma-Aldrich
- Fey SJ, Wrzesinski K. Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol Sci [Internet]. 2012;127(2):403–11.
- Ramaiahgari SC, den Braver MW, Herpers B, Terpstra V, Commandeur JNM, van de Water B, et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol [Internet]. 2014;88(5):1083–95.
- Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol
- Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Vol. 433, Biochemical and Biophysical Research Communications. 2013.
- Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, Matuschewski K, et al. Intravital Observation of Plasmodium berghei Sporozoite Infection of the Liver. Egwang T, editor. PLoS Biol. 2005 May;3(6):e192.
- Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol
- See also :
2D Versus 3D Cell Cultures from Mimetas
3D Cell Culture: A Review of Current Techniques whitepaper from Biotek
3D Cell Culture Tools and Applications From Merk