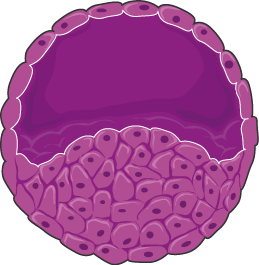
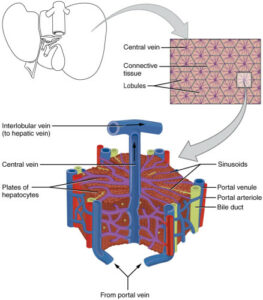
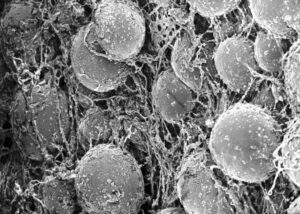
Efficacity and toxicity of candidate anticancer drugs on 3D cell culture model has been much lower than on 2D cell culture models over the past few decades. The latest models has allowed for the understanding of molecular pathways driving tumor progression and for high throughput screenings testing multiple drugs in parallel and
Despite the increasing knowledge on tumor biology, cancer remains a deadly disease, with anticancer drug candidates yielding very poor outcomes. This can be explained in part by the simplified cancer model used during drug testing, in which the effect of tumor microenvironment (TME) on tumor progression is not taken into account. Indeed, TME plays a critical role in drug resistance. Recent advances in the development of 3D in vitro models which mimic physiological conditions of human cancers have opened new venues in the anticancer drug discovery. Additionally, these in vitro models raise as an alternative to animal testing.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Role of tumor microenvironment (TME) in anticancer drug efficacity
Monolayered 2D cell culture remains the most used cell model in anticancer drug discovery. Immortalized cultured cell lines and more recently primary human tumor cell lines derived from patients have been a useful model to understand molecular pathways leading to cell proliferation. However, in vitro growth conditions in 2D cell culture are lacking important components of the tumor microenvironment (TME) [1]. The tumor microenvironment (TME) is a complex biochemical network of multiple factors surrounding tumor cells, including vascular networks, the extracellular matrix (ECM), and an heterogenous cell population composed by multiple cell types.
Complementary to 2D cell culture, in vivo xenograft mouse models (PDTX: patient-derived tumor xenograft) are widely used for anticancer drug testing. Despite being an in vivo model, the evolution of tumor in mice cannot fully recapitulate the human tumor microenvironment. Additionally, PDTX is a time and resource consuming model which doesn’t allow for high throughput screening [3].
One main cause of tumor chemoresistance to anticancer drugs is the dynamic behavior of cancer cells, with genetic and epigenetic modifications occurring over time and allowing cancer cells to escape the targeted effect. Tumor microenvironment (TME) can affect gene expression, cell metabolism, chromatin structure, cell proliferation and cell migration of both, cancer and stromal cells. Therefore, cytotoxicity of anticancer drugs is highly dependent on the TME. Because of this, results of drug testing are very sensitive to the cell culture model used. Following are some examples showing how TME can affect drug delivery and/or drug efficacity (reviewed in [1]):
– Cell density at solid tumors is not comparable to cell density in monolayered cultures. Cell density is a physical barrier which affects drug delivery.
– Changes in interstitial fluid pressure due to tumor mass growth and due the modification of composition in the extracellular matrix (ECM). Composition and stiffness of the ECM are dynamic and can influence drug intake [2]
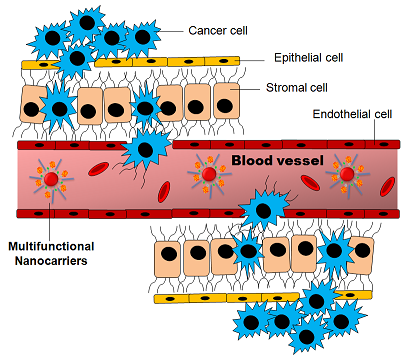
– Cell contacts between cancer cells and the extracellular matrix (ECM) can activate signaling pathways which can induce drug resistance in cancer cells, in a process termed CAM-DR (cell adhesion mediated drug resistance). Cell-cell interactions between cancer cells and stromal cells in the ECM also trigger anti-apoptotic signaling. As an example, in vitro co-culture of cancer cells with stromal cells can generate chemoresistance to drugs which are effective in mono-cultured cancer cells [1].
– Changes in vascularization in the TME can induce resistance to apoptosis. High vascularization is induced in cancer cells in a process of abnormal angiogenesis (see Scientific note Hypoxia and cancer). Angiogenesis is induced by secretion of VEGF (Vascular Endothelial Growth Factor), synthesized by tumor and endothelial cells. In addition to angiogenesis, VEGF has a role in anti-apoptotic signaling. Cancer cells can express VEGF receptors, which provide them resistance to chemotherapy [2].
– Shear stress in the TME can activate signaling pathways inducing chemoresistance.
– Hypoxic conditions: While oxygen and nutrients are unlimited in 2D cultured cells, there is a gradient of oxygen accessibility across the tumor mass in solid tumors. Hypoxic growth increases survival rates of cancer cells. Thus, mimicking oxygen conditions in in vitro assays is critical to asses drug efficacity and tumor growth.
3D cell culture to mimic tumor microenvironment
Recent advances in 3D culture technology have allowed to grow cancer and healthy tissue models in more physiological conditions which mimic the tumor microenvironment, or at least try to recapitulate some of the TME main features [5]. Some examples of these biomimetic cell culture models are:
– Spheroids: Cells are densely packed mimicking a solid tumor. This is achieved through physical confinement: enabling cell-cell interactions and preventing cell-surface interactions. Inside spheroids a gradient of oxygen concentration generates an inner quiescent zone, mimicking the conditions in a tumor mass. Consistent to the different physical properties between 2D cultured cells and spheroids, increased drug resistance to anticancer drugs has been observed in spheroids. For instance, anti-proliferative drugs initially make spheroids to shrink by affecting the outer layer, however spheroids can resume proliferation [1].
– Polymer Scaffolds: They are porous artificial matrices which mimic the ECM (Extracellular Matrix) and are composed of extracellular proteins and can include growth factors. Scaffold matrices for cell aggregation and limit oxygen availability. Cultured cancer cells or spheroids can be grown on these scaffolds, and drug resistance to anti-cancer drugs has been recapitulated in this context. For instance, a 3D scaffold mimicking the TME for an acute myeloid leukemia model showed to recapitulate phenotypes of drug resistance observed in tumors compared to traditional 2D cell cultures [7]. Therefore, 3D models are more predictive of real tumor responses.
– Organoids: They are 3D cell cultures generated from tissue-derived stem cells which can self-organize into an organoid structure. Organoids remain genetically stable over time when derived from healthy tissue stem cells. Tumor organoids have been successfully grown from patient-derived tumor tissue and they recapitulate phenotypes of morphology and behavior of the original tumors. Organotypic assemblies require a 3D matrix mimicking extracellular components. So far, organoids have been grown from multiple healthy tissues, but also from metastatic tissues and cancer biopsy samples [3]. Biobanks of organoid cancer models have been generated [4].
– Organ-on-a-chip models. A step forward in the development of organoids combines organotypic assemblies with microfluidics technology [8]. Microfluidics allows to grow cells, spheroids, or organoids in a very controlled environment inside microdevices, mimicking parameters such as blood flow, nutrient gradients, oxygen gradients or shear stress. Microfluidics also allows to model complex tissue interactions by coupling multiple organoids and by incorporating synthetic materials which recapitulate tissue-specific microenvironments.
The possibility of recreating the TME in microfluidic devices has allowed for the development of tumor-on-a-chip models [8]. Maintaining a controlled environment over time allows for monitoring tumor dynamics and the assessment of drug efficacy and resistance over time. Organ-on-a-chip and tumor-on-a-chip models are as well compatible with high-throughput screenings.
3D cell culture to mimic tumor microenvironment
Low efficacity of anti-cancer drugs is explained in part by the capability of cancer cells to adapt to a very dynamic environment. It is also explained by the lack of relevant cancer models mimicking this microenvironment. Therefore, more physiological in vitro cancer models will be crucial for the future of drug discovery.
The main advantage of monolayered cell culture in multi-well plates remains the possibility of high throughput analysis, which is a major requirement for drug screening in the pharmaceutical industry. Adaptation of 3D culture and organ-on-chip models to up-scale drug testing will be important in the coming years. Also, in vitro 3D models are able to recapitulate only a subset of features present in the tumor microenvironment, so more complex models (mimicking complex organ interactions) will be needed to closer to an in vivo model [5].
The development of 3D cell culture models has been possible due to major advances in biology (stem cell biology and genome editing techniques) and engineering (microfabrication and 3D printing, or microscopy developments to observe cells in complex environments) [5]. Despite the proliferation of these physiological models, standardized biomimetic cell culture platforms are still lacking. It is foreseeable that further technical advances in both biology and engineering will allow to implement 3D models and organ-on-chip platforms, which could eventually lead to cost-effective and patient-specific in vitro models for drug testing.
References
- [1] Jo Y. et al. Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development. Theranostics. (2018) https://www.ncbi.nlm.nih.gov/pubmed/30555545
- [2] Castells M. et al. Implication of Tumor Microenvironment in Chemoresistance: Tumor-Associated Stromal Cells Protect Tumor Cells from Cell Death. Int. J. Mol. Sci. (2012) https://www.ncbi.nlm.nih.gov/pubmed/22949815
- [3] Drost J. and Clevers H. Organoids in Cancer Research. Nature Reviews Cancer (2018). https://www.ncbi.nlm.nih.gov/pubmed/29692415
- [4] Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell (2017). https://www.ncbi.nlm.nih.gov/pubmed/29224780
- [5] Chen C.S. 3D Biomimetic Cultures: The Next Platform for Cell Biology. Trends in Cell Biology (2016). https://www.ncbi.nlm.nih.gov/pubmed/27637342
- [6] Finger EC. and Giaccia AJ. Cancer Metastasis Rev. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease (2010) https://www.ncbi.nlm.nih.gov/pubmed/20393783
- [7] Aljitawi OS. et al. A novel three-dimensional stromal-based model for in vitro chemotherapy sensitivity testing of leukemia cells. Leuk Lymphoma (2014) https://www.ncbi.nlm.nih.gov/pubmed/23566162
- [8] Sun W. et al. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Healthcare Mater (2019) https://www.ncbi.nlm.nih.gov/pubmed/30605261