FUS Liquid-Liquid Phase Separation
What is Phase Separation with FUS?
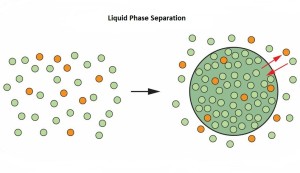
Cellular compartmentalization is essential for cell homeostasis. Cytoplasmic compartments can be delineated by molecular concentrates or condensates, generated by the physical mechanism of liquid-liquid phase separation (LLPS). One example of compartmentalization generated by molecular condensation is the one involving the protein FUS (RNA binding protein fused in sarcoma). FUS is involved in multiple cellular processes and itscellular localization, nuclear or in cytoplasmic RNP (ribonucleoprotein) granules, is important for its function. Three recent publications in the issue of Cell 173 (April 2018) uncover the molecular mechanisms controlling FUS cellular compartmentalization through LLPS.
Beyond State of the art
The Rosen and Chook labs[1] showed how the nuclear import receptor karyopherin-β2 disrupts FUS LLPS by binding to multiple sites of FUS. Additionally, Hofweber and colleagues [2] showed how a post-translational modification (arginine methylation) in FUS suppresses LLPS and mediates the nuclear import of FUS. A third publication from the Hyslop lab[3] showed that C-ter N-ter domain FUS interactions contribute to FUS LLPS and these interactions is modulated by arginine (C-ter) methylation of FUS.
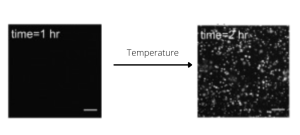
Importance of Temperature
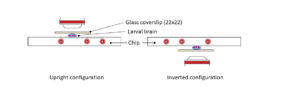
FUS liquid-liquid phase separation (LLPS) is a temperature-dependent process. In the article from Yoshizawa and colleagues [1], authors were able to shift temperature from 10°C to 44°C, at 2°C increment steps, while live imaging of the FUS condensation process. This experiment, performed with spinning disk confocal microscopy coupled to the CherryTemp temperature control system, contributed to the deciphering of the mechanism regulating FUS LLPS.
Summary
Liquid-liquid phase separation (LLPS) is believed to underlie formation of biomolecular condensates, cellular compartments that concentrate macromolecules without surrounding membranes. Physical mechanisms that control condensate formation/dissolution are poorly understood. The RNA-binding protein fused in sarcoma (FUS) undergoes LLPS in vitro and associates with condensates in cells. We show that the importin karyopherin-β2/transportin-1 inhibits LLPS of FUS. This activity depends on tight binding of karyopherin-β2 to the C-terminal proline-tyrosine nuclear localization signal (PY-NLS) of FUS. Nuclear magnetic resonance (NMR) analyses reveal weak interactions of karyopherin-β2 with sequence elements and structural domains distributed throughout the entirety of FUS. Biochemical analyses demonstrate that most of these same regions also contribute to LLPS of FUS. The data lead to a model where high-affinity binding of karyopherin-β2 to the FUS PY-NLS tethers the proteins together, allowing multiple, distributed weak intermolecular contacts to disrupt FUS self-association, blocking LLPS. Karyopherin-β2 may act analogously to control condensates in diverse cellular contexts.
Publication source
- Source of publication: Cell Press
- Paper title: Nuclear Import Receptor Inhibits Phase Separation of FUS through Binding to Multiple Sites. Yoshizawa, T., Ali, R., Jiou, J., Fung, H. Y. J., Burke, K. A., Kim, S. J., Lin, Y., Peeples, W. B., Saltzberg, D., Soniat, M., Baumhardt, J. M., Oldenbourg, R., Sali, A., Fawzi, N. L., Rosen, M. K., & Chook, Y. M. (2018)