Introduction
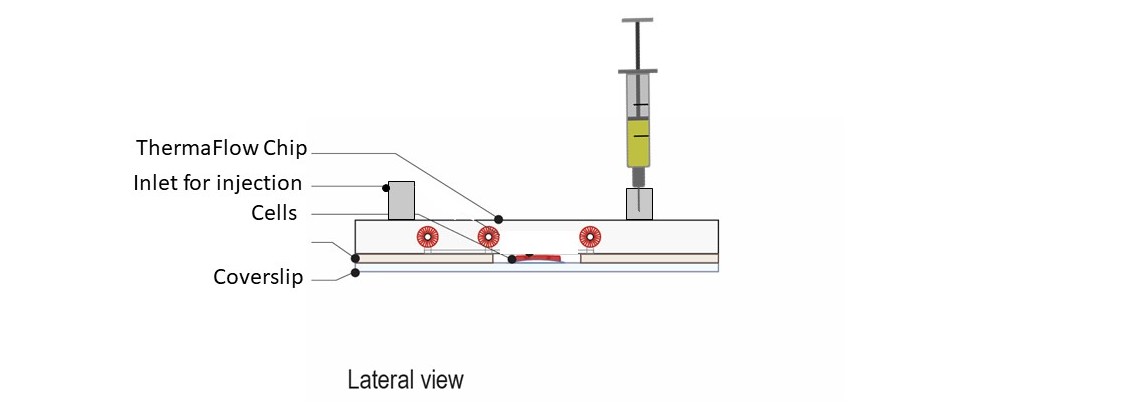
The following protocol explains how to use the temperature controller CherryTemp with the Therma Flow chip for medium perfusion when adherent mammalian cells are placed on glass coverslips. The Therma Flow chip allows for temperature control and replacement of medium and drug injection. This can be done manually using a syringe or it can be coupled to an automated pumping system for continuous perfusion. The following protocol has been optimized for HeLa-Kyoto cells. Optimization is required for different cell types.
Step-by-step guidelines:
Step 1 – Cell chamber assembly : Place a clean glass coverslip (24x60mm) on the CherryTemp dedicated stage insert. Remove the sticker from one side of the cell chamber and paste it to the glass coverslip. The insert helps to keep the coverslip and the cell chamber aligned.
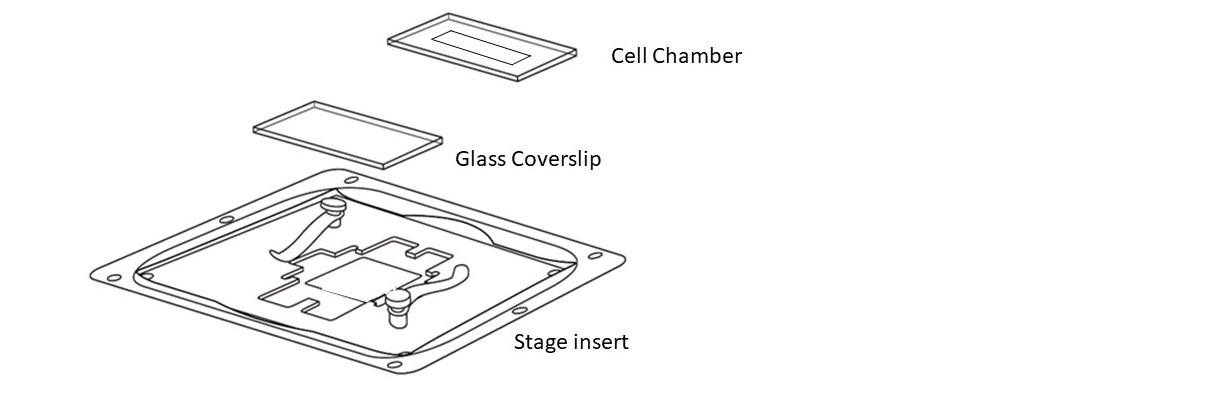
Step 2 – Sterilization : Clean the assembled cell chambers by wiping with ethanol 70%. Then place on UV light for 30 min.
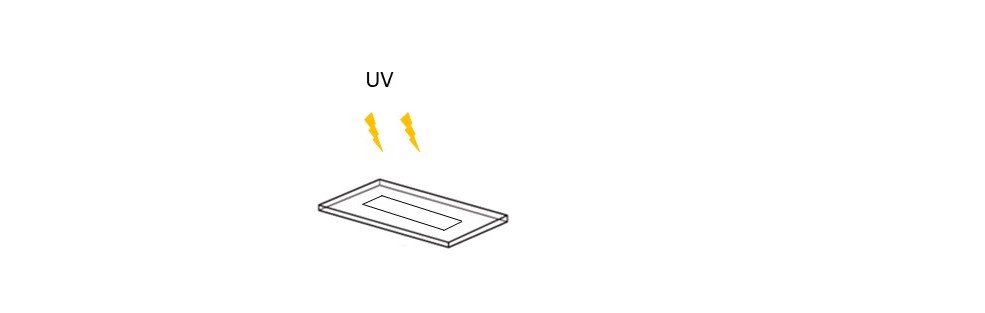
Step 3 – Cell seeding and incubation : Place 2 assembled cell chambers inside a 9cm diameter petri dish and fill in with medium (around 10mL). Concentration of cells in the Petri dish should be higher than 2.2*10¨6 cells per ml. Incubate during18H-24H until reaching a confluence of around 80% in the cell chambers.
Step 4 – Therma Flow chip assembly : Dry the seeded cell chamber on wipe paper. Next, place the chamber on the stage insert. Remove the top liner on the cell chamber using tweezers (starting at the corner). Place the Therma Flow on top and press during 1 minute to attach it.
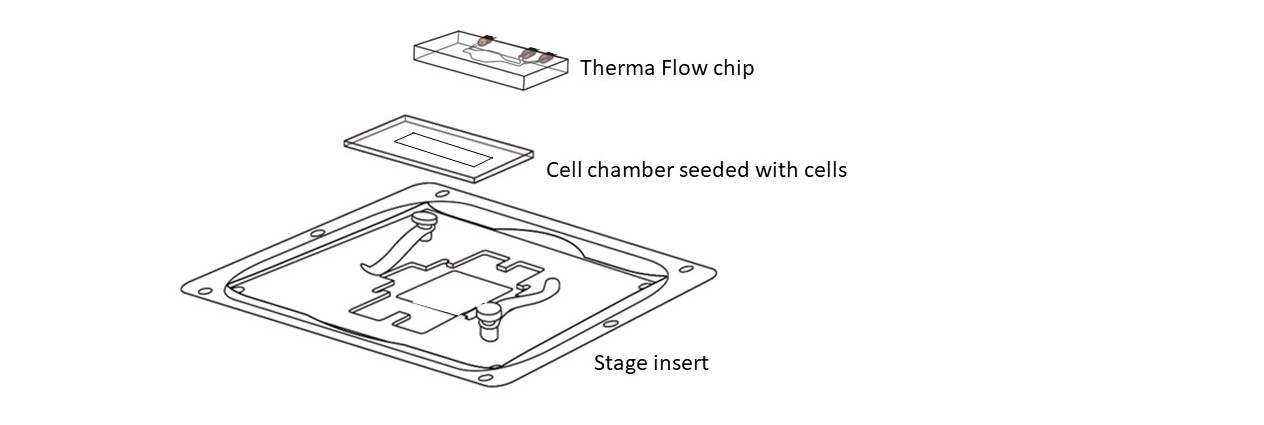
Step 5 – Medium injection : Fill the inlet of the Therma Flow with 2x200uL of Medium (DMEM plus HEPES). Connect a syringe to the outlet luer and gently vacuum until the medium in the inlet fills the cell chamber and removes all the air inside. Once the cell chamber is full of medium, add again 200uL of medium. Place the ThermaFlow inside a 15cm Petri dish to transport it to the incubator or to the microscope.