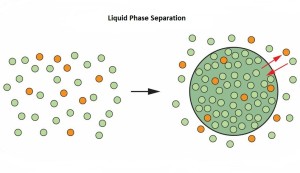
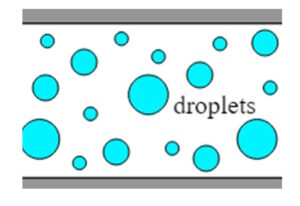
Mounting method for in vitro imaging of molecular assembly using CherryTemp
This protocol shows how to create a chamber on a glass coverslip to image in vitro biochemical reactions.
Placing a CherryTemp chip on top of this chamber allows for thermalization of the sample, to observe temperature dependent molecular dynamics in the seconds time scale.
Two types of spacers can be used:
-LSR elastomere: for volumes up to 10ul
-Silicone spacer: to create a sealed chamber for larger volumes up to 50µL
Step-by-step guidelines:
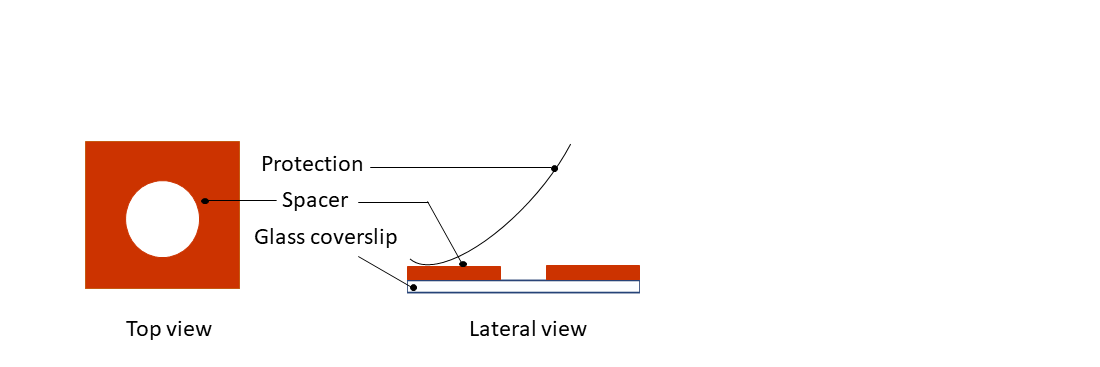
Step 1: Remove the protection from the dedicated spacer (in vitro applications, red spacer) and paste it on top a 24x24mm glass coverslip.
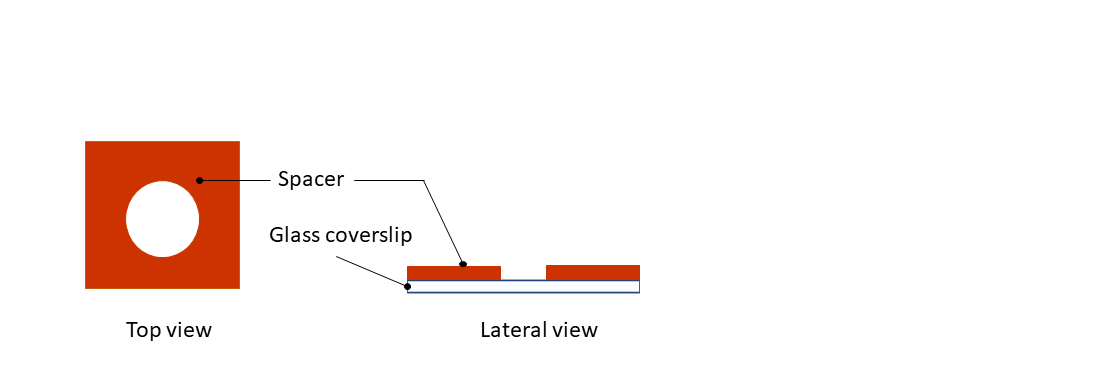
Step 2: Remove the second protection from the silicone spacer.
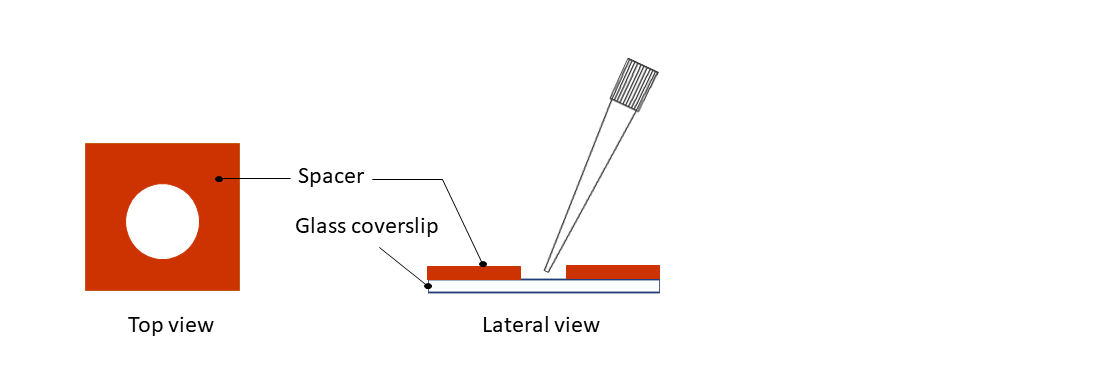
Step 3: Pipette your sample on the coverslip.
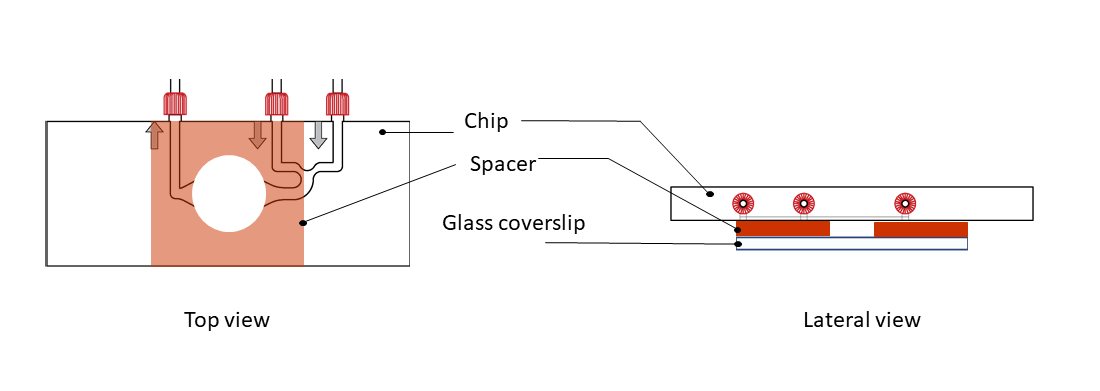
Step 4: Top the glass coverslip with the CherryTemp chip, by sticking it to the spacer. Place the chip with the correct orientation (thermalization chamber facing down, in contact with the spacer), with the thermalization chamber centered on the sample.
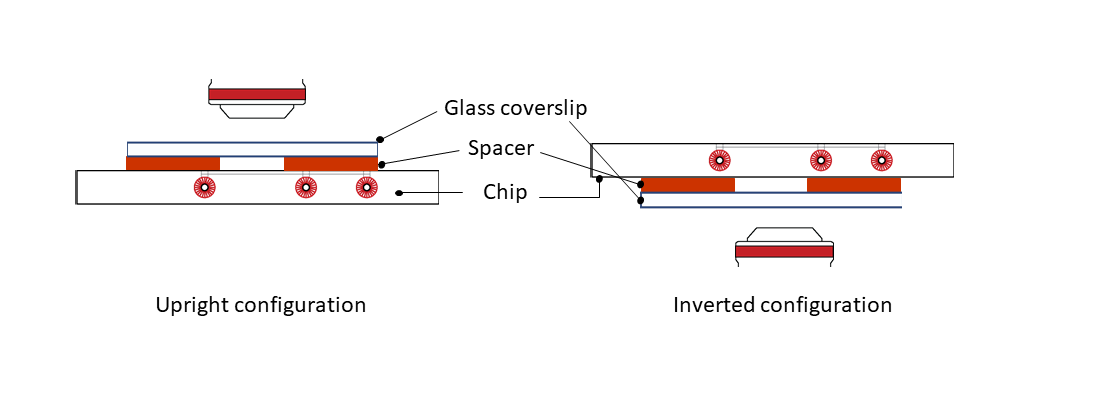
Step 5: Place the mounted system on either an upright or an inverted configuration, depending on your microscope setup.
Step 6: When finished, remove the CherryTemp chip from the coverslip
– The CherryTemp chip can be cleaned by wiping the chamber part with ethanol (don’t soak the chip on ethanol)
– The silicone spacer can be cleaned with ethanol
References
Protocol adapted from Rosen Lab, University of UT Southwestern Medical Center in Dallas.