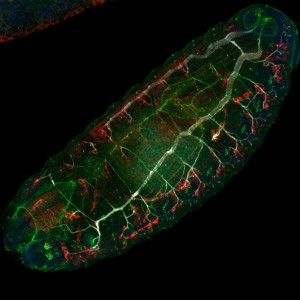
Mounting method for third larval brains of D. Melanogaster
Introduction
This protocol shows how to mount Drosophila 3d instar larval brains using a CherryTemp chip. CherryTemp allows for heating/cooling the brains while doing high resolution live microscopy.
Dissection of brains can be performed using standard protocols.
5 to 10 brains can be used for imaging.
Protocol from Paul T. Conduit lab, Institut Jaques Monod, France.
Here some videos of brains mounted with this protocol :
Conduit Imaging Drosophila third instar larval brain cells expressing EB1-GFP as a growing microtubule marker
Drophila microtubule depolymerization using the temperature
Step-by-step guidelines:
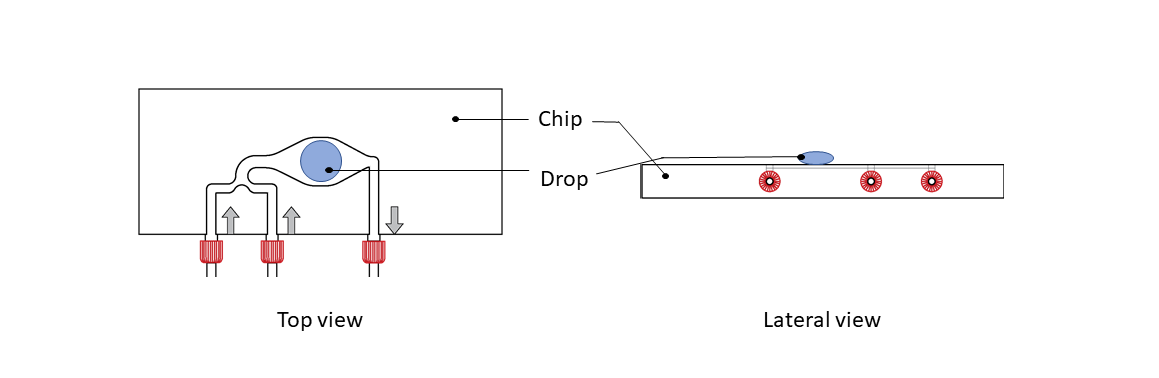
Step 1: Place a 10µl drop of Live Cell Imaging Solution (Life Technologies, A14291DJ) on top of the CherryTemp chip, centered on the thermalization pattern.
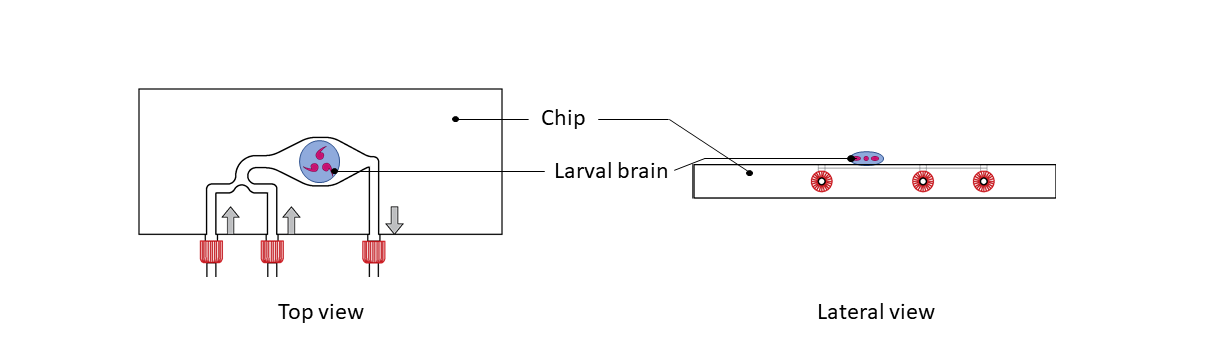
Step 2: Place dissected Drosophila brains on the drop. Brains must be centered on the thermalization pattern.
Step 3: Place A 22x22mm coverslip on top. Carefully lower the coverslip such that the brains become semi-squashed.
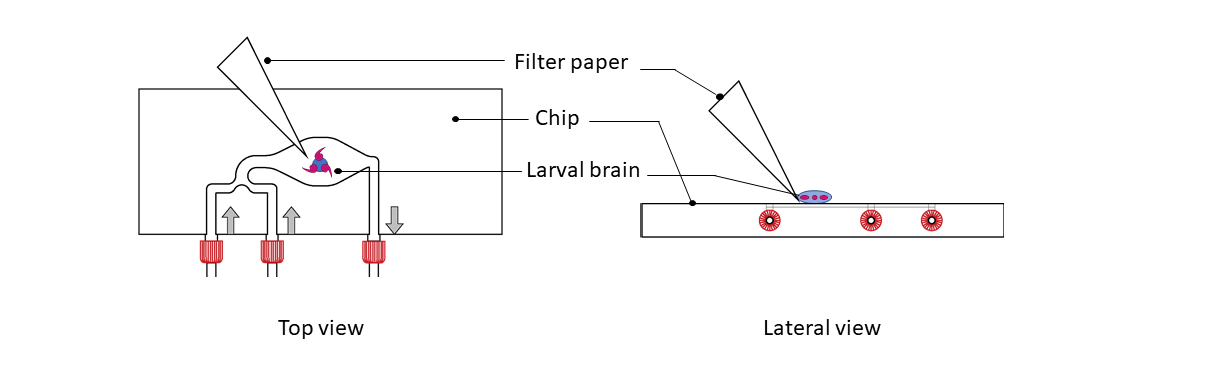
Step 4:
Use filter paper to suck excess liquid from the side of the coverslip until a reasonable number of cells had been pushed into a single layer at the edge of the brain
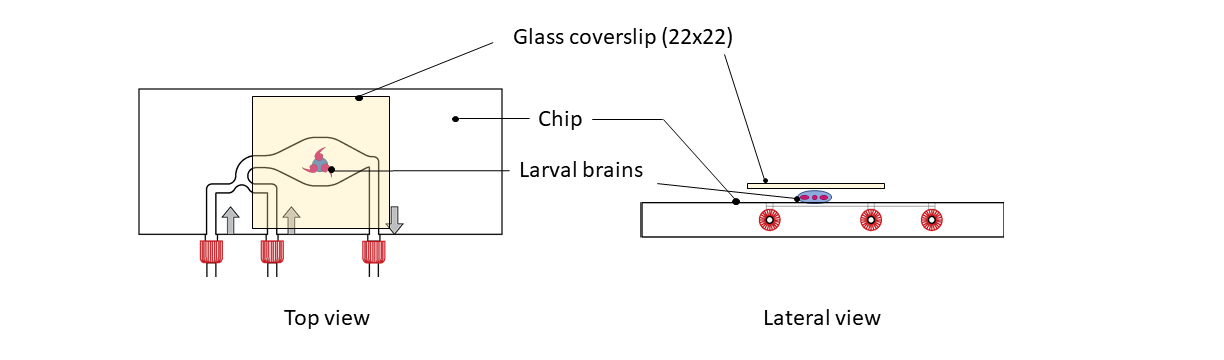
Step 5:
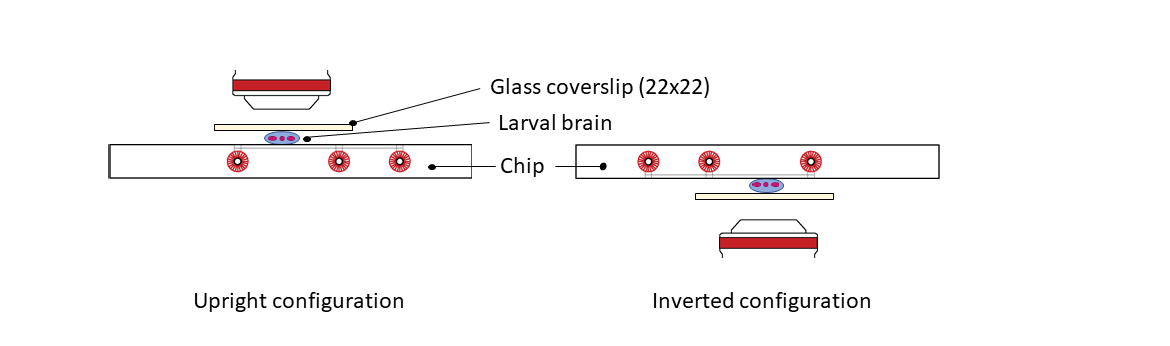
Place the mounted sample on the microscope stage insert, in an upright or an inverted configuration. For an inverted microscope, turn the mounted sample with the CherryTemp chip on top and clip the CherryTemp chip on the stage insert. Brains will remain semi-squashed and are ready for imaging.
References
Protocol from Paul T. Conduit lab, University of Cambridge.