Mounting method for Drosophila pupa
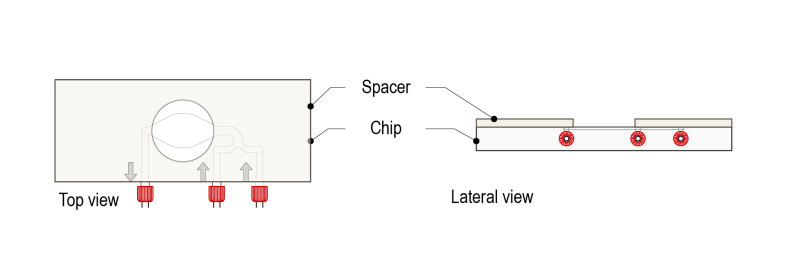
With this protocol, spacers of multiple thicknesses can be used to adjust to the diameter of the pupa. Spacers thicknesses available are 100µM, 250µM and 500µM and they can be piled up to customize chambers according to the pupal diameter. Spacers can replace standard methods using glass coverslips and halocarbon oil drops.
Note: This protocol is for short term imaging (up to 2 hours). For long-term imaging protocols spacers can be replaced by gas permeable materials.
Step-by-step guidelines:
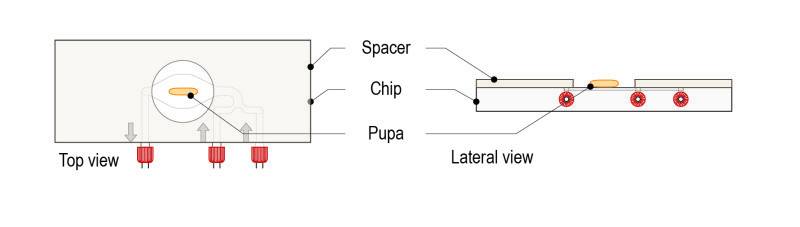
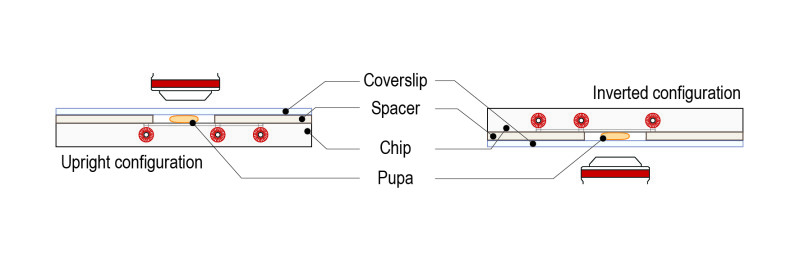
Step 1: Place the spacer on the glass surface of the CherryTemp chip (engravements facing down, coverslip and liquid microfluidic chamber facing up, in contact with the spacer).
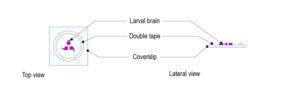
Step 2: Stick the intact pupa (ventral side down) to a microscope slide coated with double-sided tape.
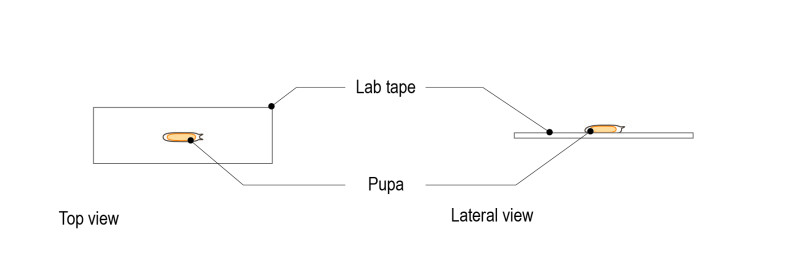
Step 3: Under the dissection microscope, gently remove the pupal case with forceps.
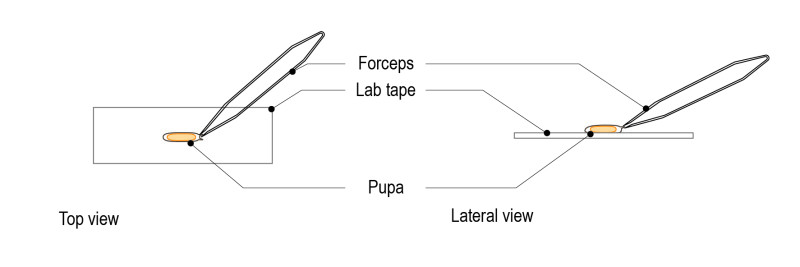
Step 4: Under the dissection microscope, carefully pick the pupa with a brush.
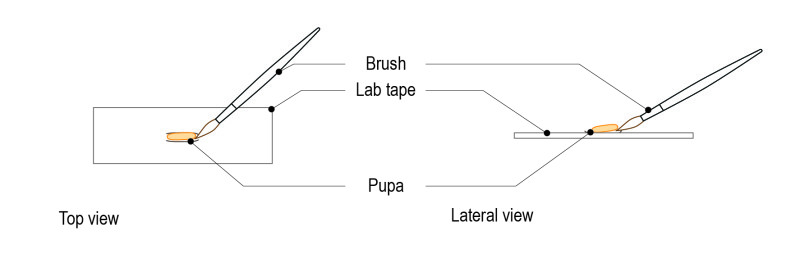
Step 5: Gently place the pupa on the glass surface of the CherryTemp chip. Place it with the desired orientation (imaging zone facing up).
Important: place the pupa centred in the chamber, which corresponds to the thermalisation zone.
.
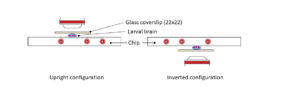
Step 6:Top with a high-resolution glass coverslip. The mounted sample is ready for observation in an upright microscope configuration.
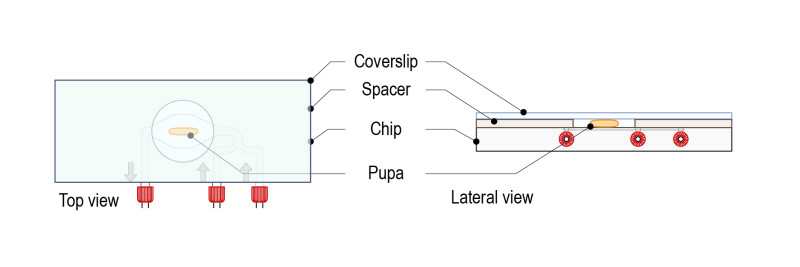
Step 7: Place the mounted slide on either an upright or an inverted configuration by simply rotating 180°.
References
Zitserman,D. and Roegiers, F. J Vis Exp 2011; (51): 2706