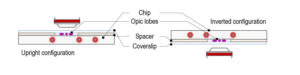
Mounting method for D. Melanogaster optic lobes
This protocol is for short term imaging (up to 2 hours) of optic lobes. Spacers can replace standard methods which use glass coverslips, glycerol and halocarbon oil drops as mounting materials. For long-term imaging protocols, spacers can be replaced by gas permeable materials.
Optic lobes of larva, pupa and adult can be imaged.
Dissection of brains can be performed using standard protocols. 5 to 10 brains can be used for imaging.
Step-by-step guidelines:
Step 1: Top a glass coverslip with our dedicated spacer (spacer for optic lobes: 200µM thickness: equivalent to the thickness of a coverslip).
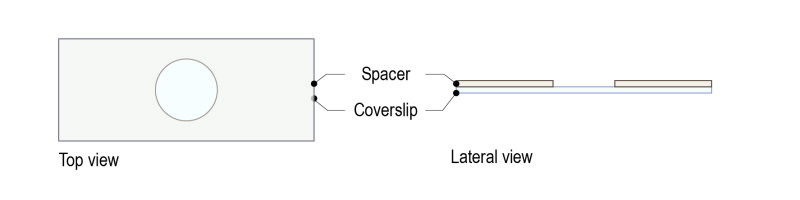
Step 2: Place a drop of 5ul of mounting medium and use forceps to place the brains. Place the medium in the spacer’s hole, without touching the sides of the spacer. Place the brains with the wanted orientation.
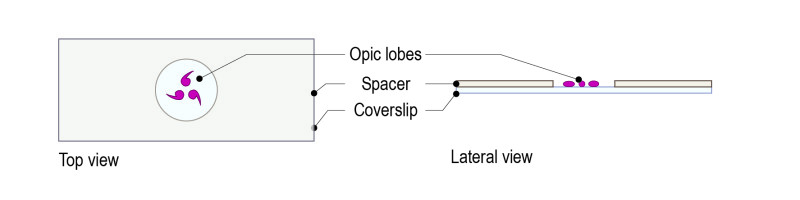
Step 3: Top the spacer with the CherryTemp chip. Place the chip with the correct orientation (thermalisation chamber facing down, in contact with the spacer). There is no need of further sealing.
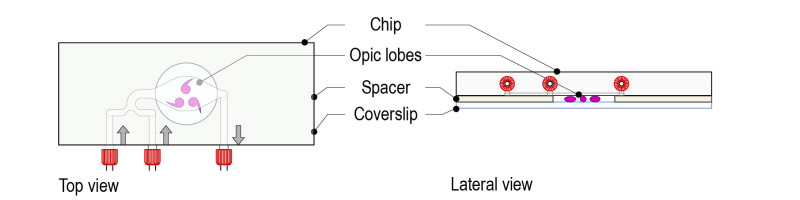
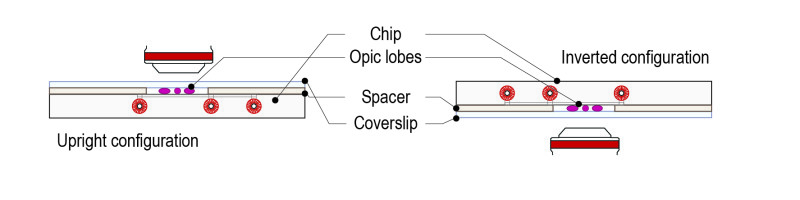
Step 4: Place the mounting system on either an upright or an inverted configuration depending on the microscope setup.
References
Drosophila Neurobiology Manual, Copyright 2010. Cold Spring Harbor Laboratory Press (Chapter 10, Morante J., Desplan, C.)