Mounting method for Drosophila larval brain
Introduction
With this protocol, a gas permeable chamber is created by means of a double-sided tape. The permeable chamber allows for a better preservation of the larval brains during imaging. The double-sided tape is porous (gas permeable) and hydrophobic (it prevents evaporation).
Time of mitosis will be an indicator of healthy brains.
Under the dissection microscope, explant CNS from third instar larvae and isolate intact brains.
Note: Fat bodies can be isolated as well to be added to the medium.
Step-by-step guidelines:
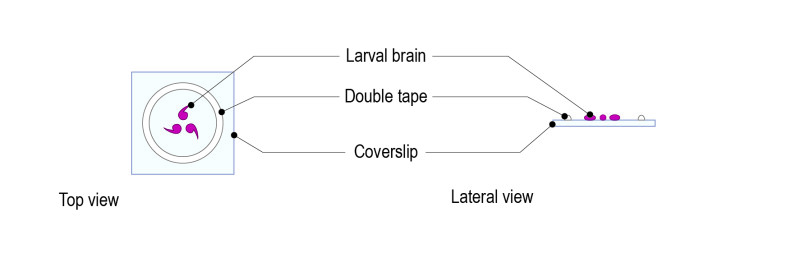
Step 1 : Stick a ring shaped double-sided tape on a square glass coverslip (high resolution, 24mm x 24mm) after removing both sides of paper from the tape. Note: optimal thickness of the double-sided tape for larval brains should be 1mm.
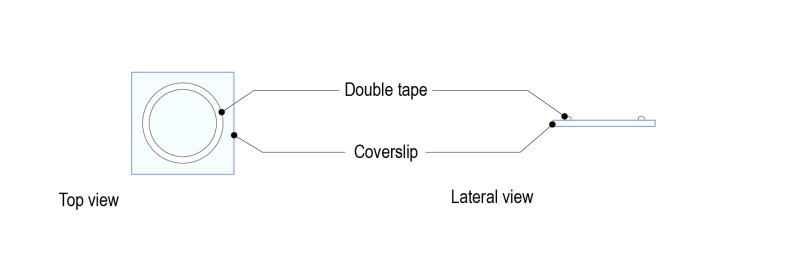
Step 2: Place a drop of medium (up to 70µl) and gently place 5 to 10 brains. Place the brains with the desired orientation: the part to be observed must be facing the glass coverslip.
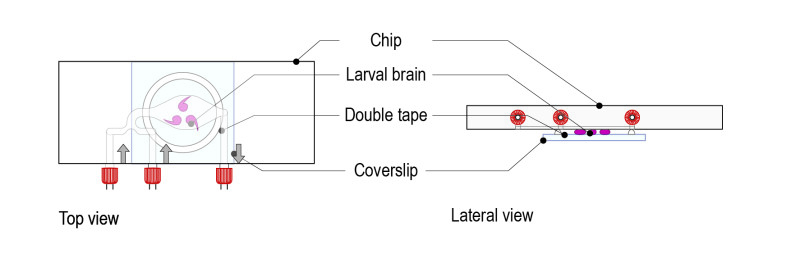
Step 3: Gently place the microfluidic thermalisation chip on top of the chamber. It will stick to the tape and the medium will disperse across the chamber. Important: The chamber must be centred on the thermalisation pattern of the microfluidic chip.
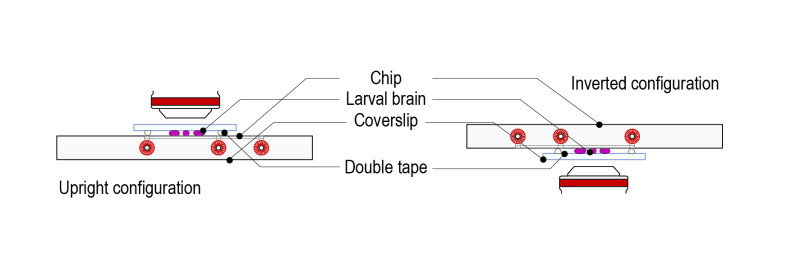
Step 4: Place the mounted slide on either an upright or an inverted configuration by simply rotating 180°.
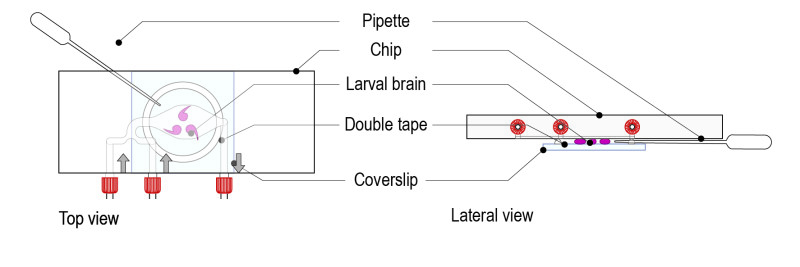
Step 5: Remove the excess of medium by pinching a Pasteur pipette through the tape ring (porous). The brains must be in direct contact with the glass coverslip. Gently move the glass coverslip to maintain the brains in the desired orientation.
Important: avoid excessive squeezing of brains (this can happen if too much medium is removed).
References
Protocol adapted for the CherryTemp technology from:
- Imaging in stainless-seel slide:
- Siller et al. Molecular Biology of the Cell Vol. 16, 5127–5140, November 2005,
- Cabernard C. and Doe C.Q. Cold Spring Harb Protoc; 2013; doi:10.1101/pdb.prot078162
- Imaging in membrane dish: Lerit et al. J. Vis. Exp 2014. (89), e51756, doi:10.3791/51756