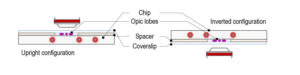
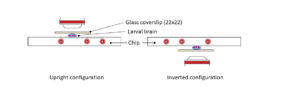
Mounting method for Drosophila larva
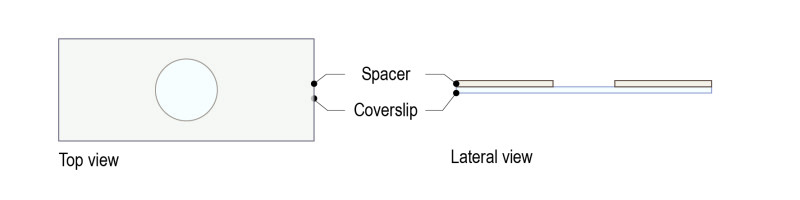
With this protocol, spacers of multiple thicknesses can be used to adjust to 1st, 2nd and 3d instar larvae. Spacers available are 100µM, 250µM, 500µM thick and they can be piled up to customize chambers of a wanted height, according to the larva diameter. Spacers can replace standard methods using glass coverslips and halocarbon oil drops.
Larvae can be anesthetized with the method of choice.
Note: This protocol is for short term imaging (up to 2 hours). For long-term imaging protocols (imaging disks, membrane dishes) spacers can be replaced by gas permeable materials.
Step-by-step guidelines:
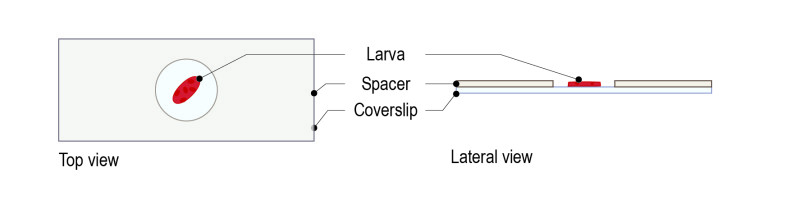
Step 1: Top a glass coverslip with our dedicated spacer (spacer for 3d instar larva: 250 µM thickness).
Step 2: Gently pick the larva with a brush and place it in the spacer’s hole with the desired orientation (area to image facing the glass coverslip).
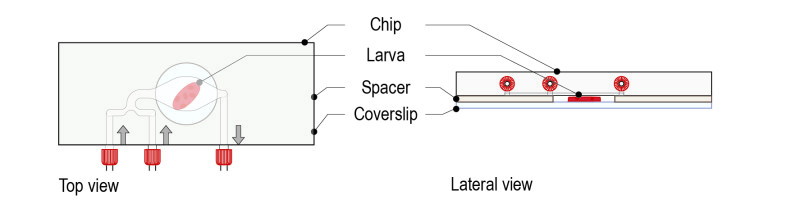
Step 3: Top the spacer with the CherryTemp chip. Place the chip with the correct orientation (thermalisation chamber facing down, in contact with the spacer). For optimal imaging, larvae must be slightly pressed, but not squeezed
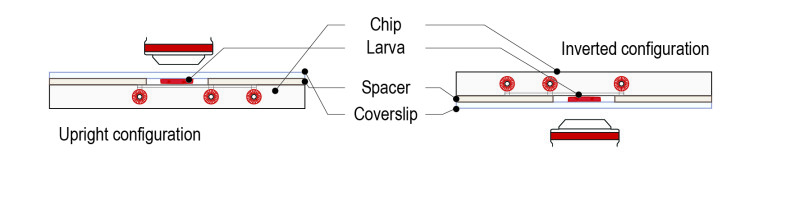
Step 4: Flip the mounted system 180°C for an upright microscope configuration
References
Protocol for the CherryTemp technology adapted from:
- Our customers experience
- Patel AA., Cox D., Bio Protoc. 2017 July 5; 7(13)
- Zhang Y., et al. J Vis Exp. 2010; (43): 2249.